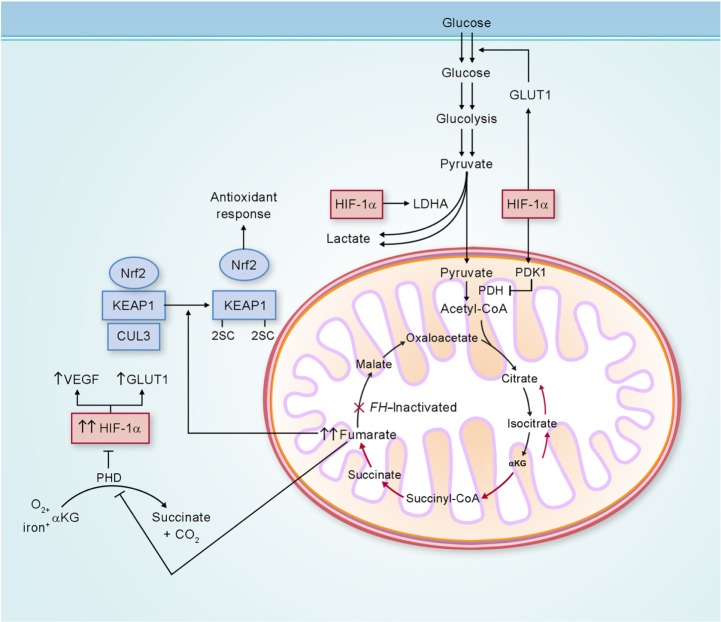
Figure 3.
Potential biochemical pathways deregulated as a consequence of FH inactivation.
Notes: FH-deficient kidney cancer has impaired oxidative phosphorylation, and undergoes a metabolic shift to aerobic glycolysis to generate adenosine triphosphate for cellular energy demands. Fumarate, which accumulates in FH-deficient cells, can competitively inhibit prolyl hydroxylase (PHD), resulting in HIF-1α stabilization and increased expression of the HIF-1α target genes VEGF and GLUT1. Elevated fumarate also succinates KEAP1, altering its conformation and inhibiting its ability to degrade nuclear factor erythroid 2-related factor 2 (Nrf2). Nrf2 transcriptional activity is upregulated, leading to activation of the antioxidant-response pathway and protection against oxidative stress. Adapted from Linehan and Rouault.21
Abbreviations: LDHA, lactate dehydrogenase A; CoA, coenzyme A; αKG, α-ketoglutarate; FH, fumarate hydratase; GLUT1, glucose transporter 1; KEAP1, kelch-like ECH-associated protein 1; CUL3, cullin 3; HIF-1α, hypoxia-inducible factor 1 alpha; VEGF, vascular endothelial growth factor; PDH, pyruvate dehydrogenase; PDK1, pyruvate dehydrogenase kinase 1.