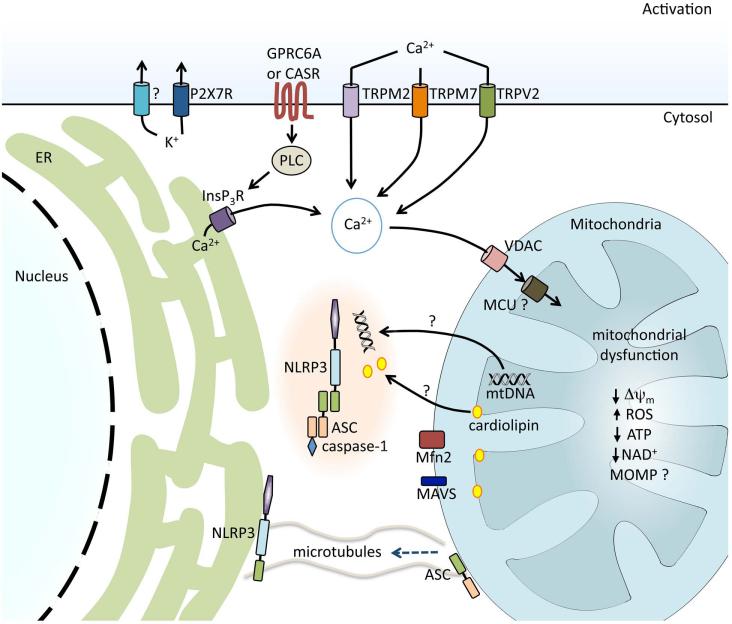
Figure 4.
Regulation of NLRP3 inflammasome activation in response to ion fluxes and mitochondrial dysfunction. K+ efflux is required for NLRP3 inflammasome activation and is achieved either directly by bacterial pore-forming toxins, such as nigericin, or indirectly via receptors such as the purinergic receptor for ATP, P2X7R. During the regulatory volume decrease response to cell swelling, Ca2+ fluxes can be regulated by the transient receptor potential receptors TRPM7 and TRPV2. Ca2+ influx can also be mediated via the ROS-sensitive TRPM2. G protein–coupled receptors CASR and GPRC6A are activated by sensing extracellular Ca2+ and trigger phospholipase C (PLC), generating inositol-1,4,5-triphosphate (InsP3) and triggering the release of Ca2+ from the endoplasmic reticulum (ER). Ca2+ acts on the mitochondria via voltage-dependent anion channels (VDAC) and the mitochondrial Ca2+ uniporter (MCU), resulting in mitochondrial dysfunction. Decrease in mitochondrial membrane potential (Δψm), generation of mitochondrial ROS, decrease in ATP and NAD generation, and, possibly, mitochondrial outer membrane permeabilization (MOMP) are associated with NLRP3 inflammasome activation. Mitochondrial dysfunction and a reduction in NAD+ result in microtubule-driven apposition of ASC on the mitochondria to NLRP3 on the ER. Mitochondrial DNA (mtDNA) and the inner mitochondrial membrane lipid cardiolipin have been shown to bind to NLRP3 and induce its activation. Association between NLRP3 and mitochondrial antiviral signaling protein (MAVS) and NLRP3 and mitofusin 2 (Mfn2) have also been implicated in effective NLRP3 inflammasome activation.