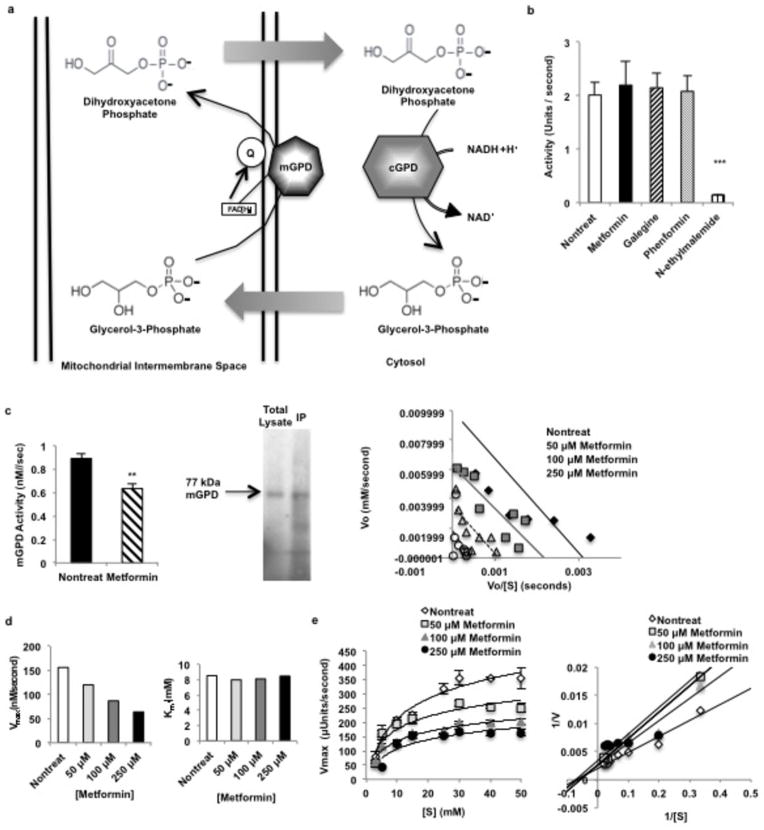
Extended Data Figure 5.
Effect of metformin on the glycerophosphate shuttle. NADH made in the cytosol via glycolysis cannot cross the mitochondrial membrane and contribute electrons to the electron transport chain (ETC) for ATP synthesis. Two mechanisms, the reversible malate-aspartate shuttle, and (a) the unidirectional glycerophosphate shuttle, oxidize NADH in the cytosol and transport electrons into the mitochondria via metabolic intermediates. The glycerophosphate shuttle is composed of cytosolic and mitochondrial glycerophosphate dehydrogenases, two structurally distinct enzymes. (b) Metformin had no effect on cGPD, which consists of two subunits and catalyzes the conversion of dihydroyacetone phosphate (DHAP) to glycerol-3-phosphate (G-3-P), oxidizing one NADH. (c) Metformin inhibited the activity of rat mGPD, a FAD+-linked enzyme that transmits electron pairs to the ETC via the quinone pool, purified from liver by immunopreciptation. Inhibition of rat mGPD was non-competitive. Data shown are the average of 5 separate experiments. (d) Metformin inhibited pure, recombinant human mGPD non-competitively, decreased Vmax without affecting Km. Data shown are representative of 2 experiments. (e) Metformin also inhibited the activity of the bacterial mGPD isoform, Pediococcus sp. α-glycerophosphate oxidase, showing non-competitive kinetics. Data are mean ± SEM, (n=4–5 technical replicates). * P<0.05, **P<0.01, ***P<0.001 by unpaired t-test.