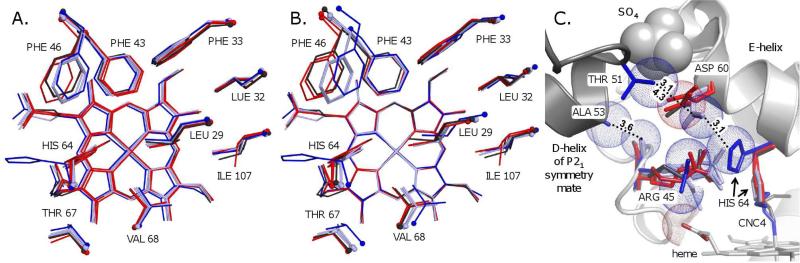
Figure 4.
The effect of the crystal form (P21 and P6) on the distal pocket structures of MbCO and MbCNC4. A. The binding pocket residues and heme groups of Mb structures aligned by all protein Cα atoms, including native Mb (P21 crystals, pH ~7.0) complexed to CO (light blue; PDB IDs 1bzr, 1vxf and 1mbc) and CNC4 (dark blue; this work), and wt Mb (P6 crystals, pH 9.0) complexed to CO (red; PDB IDs 2mgk and 1jw8) and CNC4 (black; this work). The ligands are not shown. B. The same structures were aligned by superimposing the atoms of the heme plane. This alignment emphasizes crystal lattice-dependent variations in the positions of the distal pocket amino acid side chains relative to the ligand heme complex. C. Intermonomer contacts and sulfate binding at the CD-corner of Mb that are present in P21 but not P6 crystals. The structures, colors and alignment are as in panel A. Spheres are shown with van der Waals radii for the native MbCNC4 Thr51-Cγ; Asp60-Cγ, Ala53-Cβ, His64-Cε, Arg45-O', -Nη1 and -Nη2, and sulfate atoms, and for the wt MbCNC4 Asp60-Cγ and heme propionate O1D atoms.