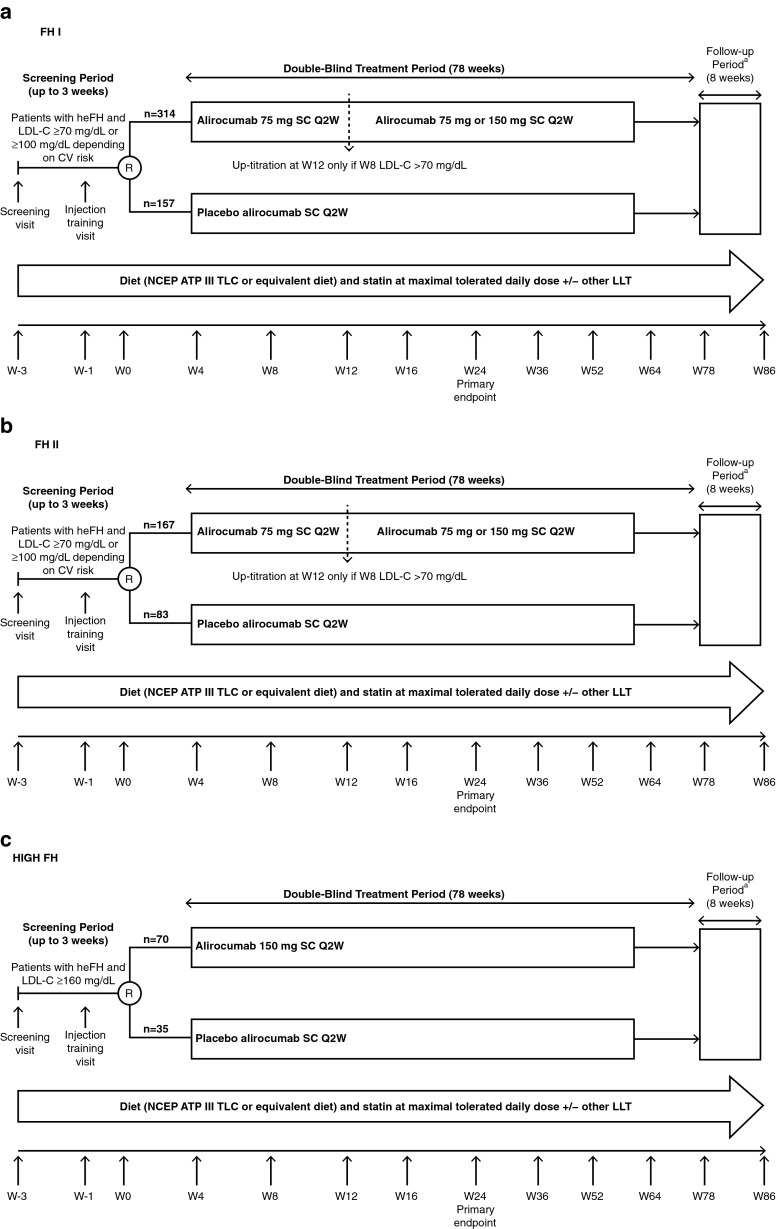
Fig. 1.
Study designs. a ODYSSEY FH I, b ODYSSEY FH II and c ODYSSEY HIGH FH. CV cardiovascular, heFH heterozygous familial hypercholesterolemia, LDL-C low-density lipoprotein cholesterol, LLT lipid-lowering therapy, NCEP ATP III TLC National Cholesterol Education Program Adult Treatment Panel III Therapeutic Lifestyle Changes, Q2W every 2 weeks, R randomization, SC subcutaneous. aAt the end of the double-blind treatment period, patients will be offered the possibility to enter an open-label extension study, in which they will receive alirocumab. If patients opt out of entering the open-label treatment period, they will enter the 8-week follow-up period