Figure 2. Chemical synthesis and folding/unfolding of disulfide-deficient analogs.
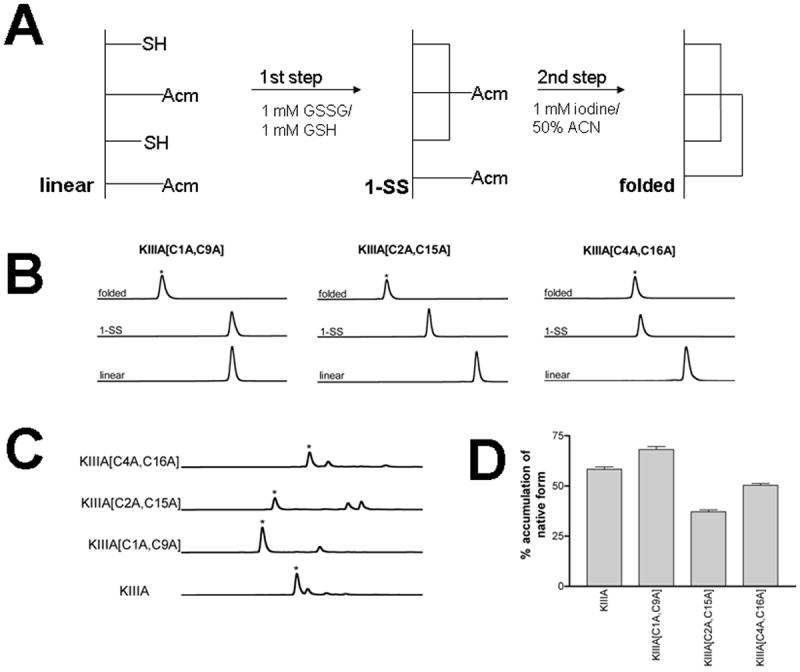
(A) - Two step oxidative folding reactions of KIIIA analogs were carried out in 0.1M Tris-HCl, pH 7.5. The first step was in the presence of 1mM GSSG and 1mM GSH. This was followed by a second oxidation step in 1mM iodine for 10-15 min. Each reaction was quenched with 8% formic acid. (B) - Oxidative folding of KIIIA analogs monitored by reversed-phased HPLC. Elution profiles of fully folded (native), first step folded, and linear forms of each analog are shown. The asterisks signify the peaks of the native forms deduced by mass spectroscopy; (C) - Reductive unfolding of the disulfide-deficient analogs of μ-KIIIA. The folded species were incubated at 25 °C in the presence of 1mM reduced and 1mM oxidized gluthathione at pH 7.5. Shown are HPLC profiles for each analog of the steady-state unfolding reaction (quenched after 30 min). The asterisks signify the native form of the analogs. (D) - Accumulation of the native form in the reductive unfolding reaction at steady-state. Average and s.e. of three independent experiments are represented.
