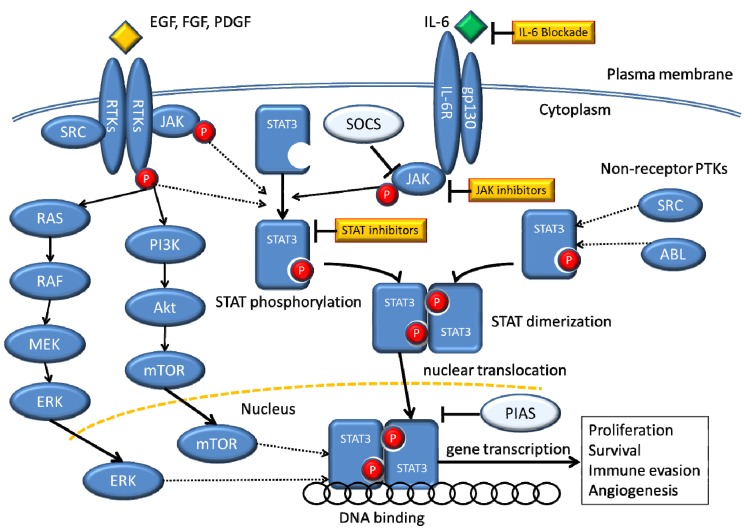
Figure 1.
Signaling pathways that converge on STAT3.
STATs are activated by receptor and non-receptor tyrosine kinases through several mechanisms. The receptor-associated Janus family tyrosine kinases (JAKs) are activated by cytokine-receptor binding through cross-phosphorylation, and then the activated JAKs phosphorylate tyrosine residues within the receptor molecules, which become docking elements for cytoplasmic STATs. STATs are subsequently phosphorylated on a single tyrosine residue and form dimers through reciprocal interaction between the phosphotyrosine of one STAT molecule and the Src homology-2 (SH2) domain of another. Dimerized STATs translocate to the nucleus to induce target gene transcription by binding to specific regulatory elements. Receptors with intrinsic tyrosine kinase activity (RTKs), including the platelet-derived growth factor receptor (PDGFR) and the epidermal growth factor receptor (EGFR), may directly activate STATs without activation of JAKs. STATs can be phosphorylated by constitutively active non-receptor protein tyrosine kinases (PTKs), such as Src and Bcr-Abl. Abbreviation: PTK, protein tyrosine kinase; IL-6, interleukin-6; P, phosphorus; SOCS, suppressor of cytokine signaling; PIAS, protein inhibitor of activated STAT.