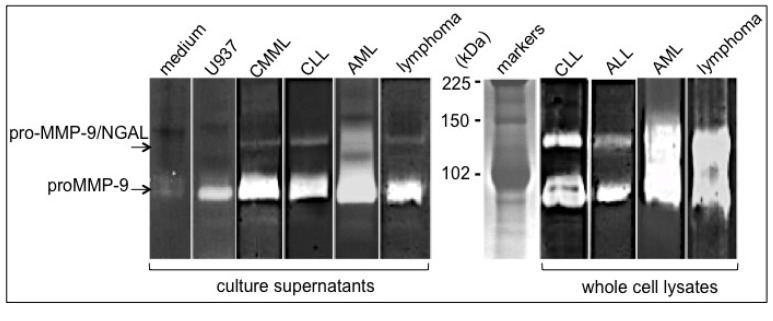
Figure 1.
Detection of pro-MMP-9 and pro-MMP-9/NGAL levels in hematopoietic malignant cells. Blood samples were obtained from patients with chronic myelomonocytic leukemia/CMML, chronic lymphocytic leukemia/CLL, acute lymphoid leukemia/B-ALL, acute myeloid leukemia/AML and Burkitt’s lymphoma. Peripheral blood mononuclear cells were separated by Ficoll-Hypaque density gradient centrifugation, washed twice in PBS, lysed or cultured as described in [38]. Whole cell lysates were obtained by lysing freshly isolated cells in M-PER buffer (4 × 106 cells/30 μL) supplemented with protease and phosphatase inhibitor cocktails as described in [38]. As a positive control for pro-MMP-9 release, U937 cells (ATCC CRL-1593.2), cultured as described in [39] were stimulated with 100 U/mL recombinant TNF-α for 48 h (R&D). The 48 h-culture supernatants from U937 cells (2 × 105/mL) and primary leukaemia cells (2 × 106/mL) were harvested by centrifugation and frozen until zymography. Control medium alone was incubated under the same conditions. Analysis of (pro)MMP-9 and NGAL presence in culture supernatants (30 μL) and whole cell lysates (30 μL) was carried out in 7.5% (w/v) SDS-PAGE containing 0.1% gelatin (w/v) as described elsewhere [18]. Zymograms showed two major bands of 130 kDa and 92 kDa corresponding respectively to pro-MMP-9/NGAL and pro-MMP-9. The sizes were determined by interpolation from a standard curve of Rf values of known molecular weight markers.