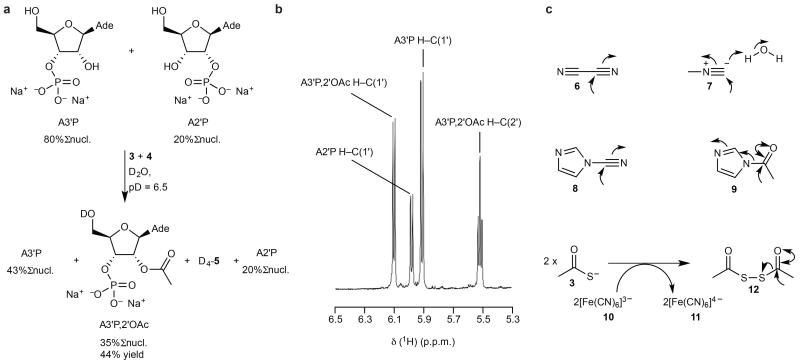
Figure 2. Chemoselective acetylation: mixtures and alternative electrophiles.
a, Treatment of A3′P (80 mM) and A2′P (20 mM) as above (Fig. 1b) results in the exclusive 2′-acetylation of the former nucleotide. b, Partial 1H-NMR spectrum of the reaction products described in a. c, Additional electrophiles (6-8) shown to drive the acetylation of ribonucleotides with thioacetate 3. Direct acetylation with 9 is also possible, as is oxidative activation of 3 with ferricyanide 10 to afford ferrocyanide 11 and a dimeric acetylating agent 12. Curly arrows indicate electrophilic activation/acetylation steps. Ade, N9-linked adenine; %Σnucl., yields expressed as percentage of total nucleotide, otherwise yield of product expressed as a percentage of the specific starting material from which it derives (as judged by 1H-NMR integration).