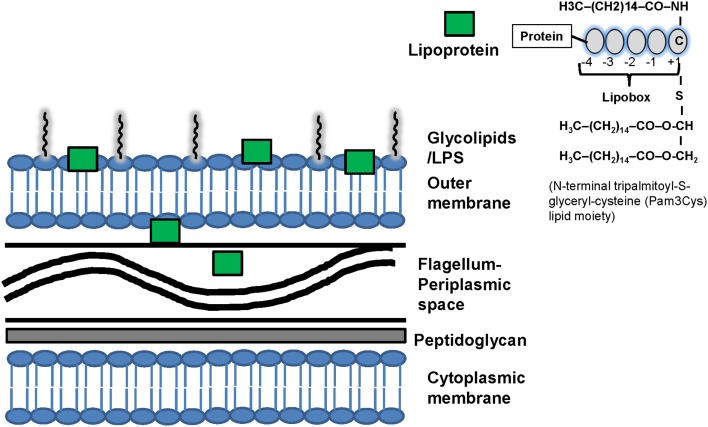
Figure 1.
Structure of spirochetal membrane and lipoproteins. Similarly to gram-positive bacteria, the spirochetal cytoplasmic membrane is associated with the cell wall that consists of peptidoglycan. Similarly to Gram-negative bacteria, spirochetes also have an outer membrane, which is not attached to the peptidoglycan layer. Spirochetes differ phylogenetically from Gram-negative bacteria and interact with the host through various structural components such as lipopolysaccharides (LPS), surface lipoproteins and glycolipids that are present mostly in the outer membrane. LPS has not been identified in Borrelia and Treponema. The periplasmic space contains the flagellum. The distribution of lipoproteins varies among spirochetes and they may be present in different cellular compartments: the outer membrane, the extracellular and the periplasmic spaces. For example the pro-inflammatory lipoproteins of T. pallidum are located below its cell surface and thus do not interact directly with the immune system of the host. It has been suggested that uptake and degradation of T. pallidum releases lipoproteins and allows their interaction with receptors on immune cells leading to immune cell activation. Computational programs can predict spirochetal protein lipidation but do not determine the location of lipoproteins in the cells. Recently, developed fluorescence activated cell sorting (FACS) and surface proteolysis methods can be used to screen for lipoprotein localization. Right upper corner: structure of spirochetal lipoproteins. The finding of a cysteine residue after a signal peptide (+1) is suggestive evidence that a protein is lipidated. The spirochetal lipoproteins have a lipobox that is four amino acids in length and mediates NH2-terminal lipidation on a conserved cysteine residue. Lipoproteins interact with the phospholipids of membranes via three hydrophobic N-terminal acyl moieties (often palmitate; C16) attached to a N-terminal cysteine residue which may contribute to the localization of spirochetal lipoproteins. An analysis of the fatty acids of T. pallidum, B. burgdorferi, L. interrogans phospholipids and lipoproteins found that while fatty acids with different length side chains (C16 and C18) were found in phospholipids, palmitate (C16) predominated in the lipoproteins. The N-terminal tripalmitoyl-S-glyceryl-cysteine (Pam3Cys) lipid moiety is the part of the lipoprotein that confers its immunologic activity. C, cysteine; LPS, lipopolysaccharides.