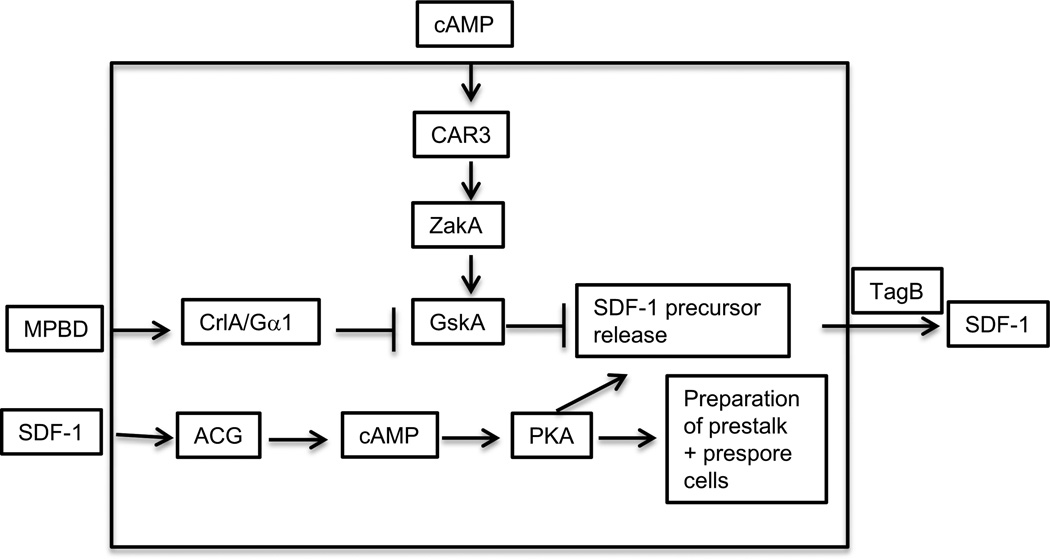
Figure 10.
SDF-1 signaling. The small phosphopeptide SDF-1 is secreted after 18 hours of development and signals both prestalk and prespore to prepare themselves for terminal differentiation. This process requires both RNA and protein synthesis. SDF-1 is cleaved from a larger phosphoprotein that is secreted in response to signaling by a polyketide, MPBD, or an increase in the activity of PKA. MPBD is synthesized by the Steely protein, StlA, and binds to the GPCR receptor CrlA. The signal is transduced by the trimeric protein that contains the Gα1 subunit such that the protein kinase GskA is inhibited and no longer blocks release of the SDF-1 precursor protein. The precursor is processed into SDF-1 by the extracellular protease domain of the prestalk specific protein TagB. SDF-1 stimulates PKA activity in a process that depends on the adenylyl cyclase ACG. High PKA activity can also trigger release of the SDF-1 precursor protein such that low levels of SDF-1 are amplified by "priming'.