Figure 3.
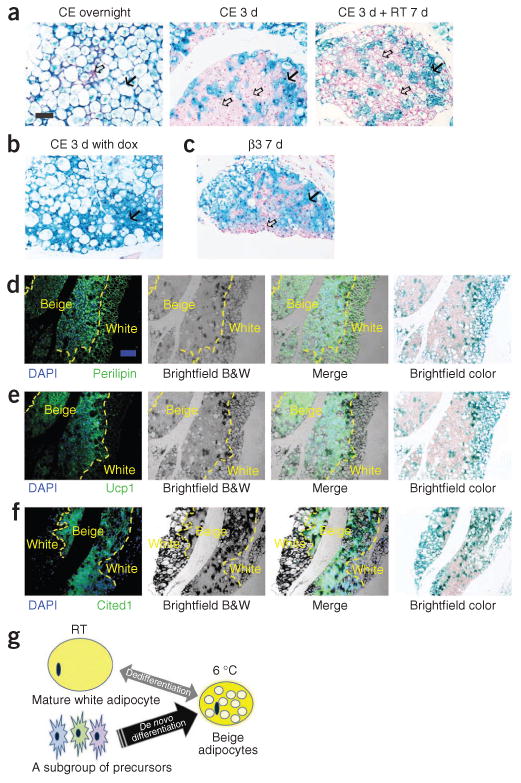
Lineage of the brown-like adipocytes in subcutaneous adipose tissue after cold exposure. (a) Representative β-gal staining of sWAT from 10-week-old male AdipoChaser mice that were kept on doxycycline diet for 7 d followed by chow diet for 3 d and then exposed to cold (CE) overnight (left) or for 3 d (middle) or exposed to cold for 3 d followed by 7 d in room temperature (RT; right). (b) Representative β-gal staining of sWAT from 10-week-old male AdipoChaser mice that were on doxycycline diet before and during 3 d of cold exposure as a positive control group. (c) Representative β-gal staining of sWAT from 10-week-old male AdipoChaser mice on doxycycline diet for 7 d followed by chow diet for 3 d and then given 7 d of daily β3 agonist treatment. Solid arrows (a–c), LacZ-positive cells; open arrows (a,c), LacZ-negative cells. Scale bar (shown in a, applies to a–c), 100 μm. For a–c, n = 3 male mice per group. (d–f) Immunofluorescence staining for perilipin (green) (d), Ucp1 protein (green) (e) or Cited1 protein (green) (f) on slides prestained with β-gal. The male AdipoChaser mice in d–f were pretreated with doxycycline diet and exposed to cold for 3 d on a chow diet. Blue indicates pre-existing white adipocytes. Scale bar (shown in d, applies to d–f), 200 μm. The yellow dashed outlines indicate the borders between white and beige adipocytes. For d–f, n = 2 male mice per group. B&W, black and white. (g) Schematic model showing that the beige cell population arises predominantly from de novo adipogenesis rather than transdifferentiation. After cold exposure or β3 agonist treatment, most beige adipocytes are induced by differentiating from cell populations other than existing mature adipocytes (beige precursors) rather than through dedifferentiation of mature white adipocytes.
