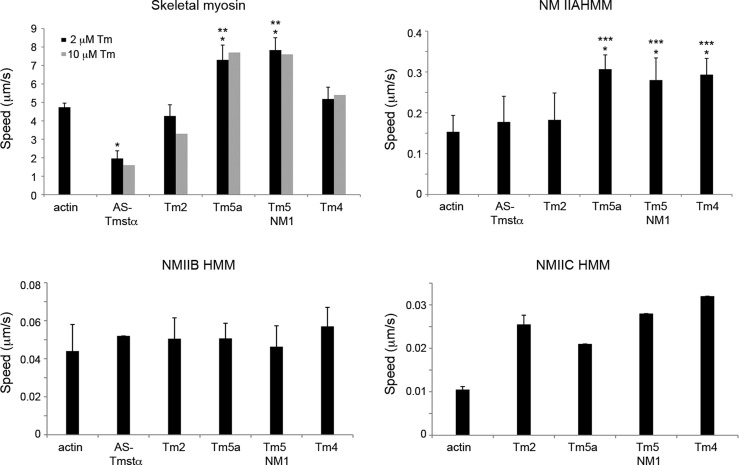
Figure 2.
Filament speeds of actin–tropomyosin in in vitro motility assays. Filament speeds were determined for actin and actin–Tm on skeletal myosin and phosphorylated NMIIA, NMIIB, and NMIIC HMMs. The assays with actin–Tm were conducted with 2 μM Tm (black bars). Assays were also conducted with 10 μM Tm for skeletal myosin (gray bars). The filament speeds are means ± SD from two to six experiments (Table 1). The data with no error bars are from a single experiment. *P < 0.01 compared with actin. **P < 0.01 compared with AS-Tmstα, Tm2, and Tm4. ***P < 0.05 compared with AS-Tmstα and Tm2 (unpaired Student’s t test). Assay conditions: 25 mM imidazole (pH 7.6), 25 mM KCl, 4 mM MgCl2, 7.6 mM MgATP, 50 mM DTT, 0.5% methyl cellulose, and an oxygen scavenger system (0.1 mg/mL glucose oxidase, 0.02 mg/mL catalase, and 2.5 mg/mL glucose). The temperature was 27 °C for skeletal myosin and 32 °C for HMMs IIA, IIB, and IIC.