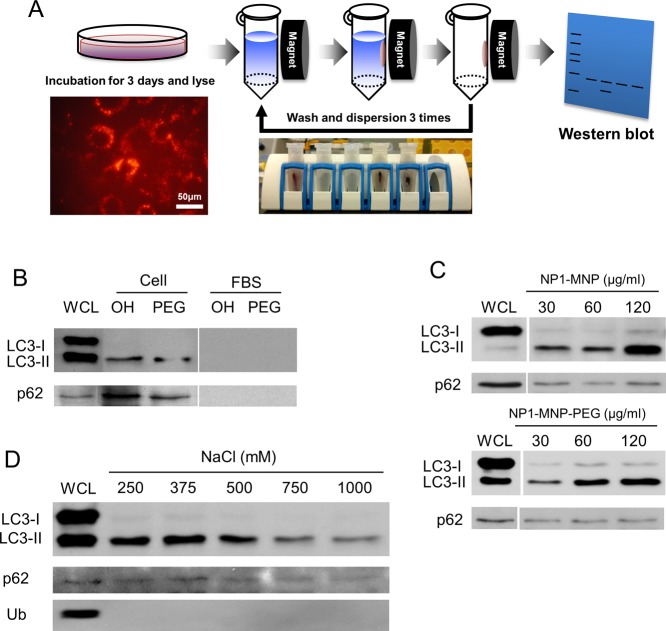
Figure 4.
Nanoparticle “pulldown” assays identify interaction with autophagy proteins. The metal core of NP1-MNP was used to “pulldown” nanoparticles and any associated proteins with a strong magnet. (A) Schematic of the assay that leverages the magnetic core to separate particles from lysate and identify bound proteins by Western blotting. Cells are incubated with NP1-MNP or NP1-MNP-PEG (100 μg/mL) for 3 days, lysed, and collected by magnetic separation. NP1-MNP is washed three times using the same approach, and bound proteins are analyzed by SDS-PAGE separation and Western blotting. (B) MC3T3-E1 cells were treated as described in (A), and the resulting blot probed for p62 and LC3β. NP1-MNP “OH” and NP1-MNP-PEG “PEG” were also incubated with fetal bovine serum “FBS” as a control for nonspecific binding. Whole cell lysate “WCL” was also run for comparison. LC3-II protein was dose-dependently, surface-independently separated by the magnetic pulldown method. (C) The pulldown assay was performed as in (A) with cell incubated with increasing concentrations of NP1-MNP and NP1-MNP-PEG, as indicated. (D) The pulldown assay was performed as in (A) with NP1-MNP-PEG, and the stringency of the wash conditions was increased by increasing salt concentrations. Representative of three independent experiments.