Figure 1.
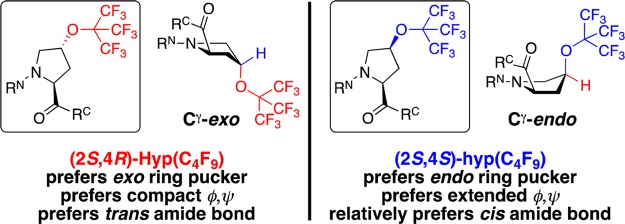
(2S,4R)- and (2S,4S)-perfluoro-tert-butyl 4-hydroxyprolines and their expected conformational preferences, in which the 4-substituents are in a pseudoaxial position on the pyrrolidine ring due to stereoelectronic effects of the 4-substitution, based on data in Ac-TYXN-NH2 peptides (X = 4-substituted proline).42 Hyp = 4R-substituted (trans relative stereochemistry) hydroxyproline, indicated by use of capitalized three-letter code and red color; hyp = 4S-substituted (cis relative stereochemistry) hydroxyproline, indicated by lower-case three-letter code and blue color. Proline exhibits a mixture of exo and endo ring puckers. RN and RC indicate the N-terminal and C-terminal peptide sequences, respectively.
