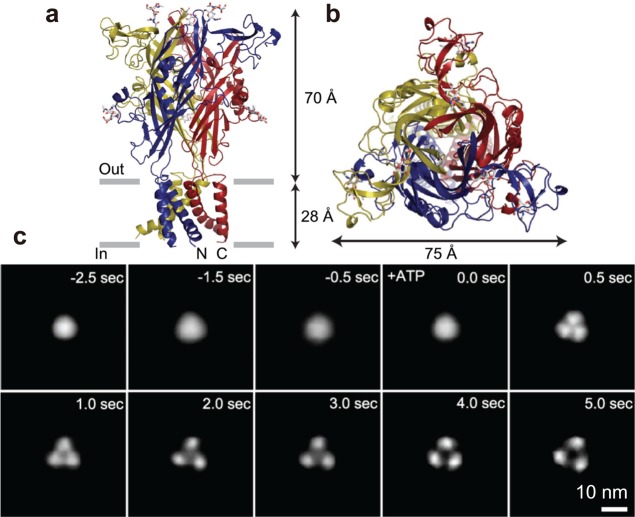
Figure 28.
Structure of P2X4 receptor. (a) Atomic structure of N and C termini deletion mutant of homotrimeric P2X4 receptor viewed parallel to the membrane. Each subunit is depicted in a different color. The gray bars suggest the boundaries of the outer (out) and inner (in) leaflets of the membrane bilayer. (b) Atomic structure of N and C termini deletion mutant of homotrimeric P2X4 receptor viewed from the molecular 3-fold axis (from the extracellular side). Reprinted with permission from ref (232). Copyright 2009 Nature Publishing Group. (c) Successive AFM images showing ATP-induced structural changes of P2X4 receptor. Before activation, P2X4 receptor was in circular shape and exhibits some fluctuation (−2.5 to ∼0.0 s). Caged ATP (200 μM) was uncaged at 0 s, after which the structure of P2X4 receptor changed to a trimer structure within 0.5 s. P2X4 receptor then exhibited a further structural change and adopted a pore dilation-like conformation. Note that the images are not directly captured ones but are those obtained by averaging images of 10 P2X4 receptor particles to reconstruct 3-fold symmetric images at given elapses of time.