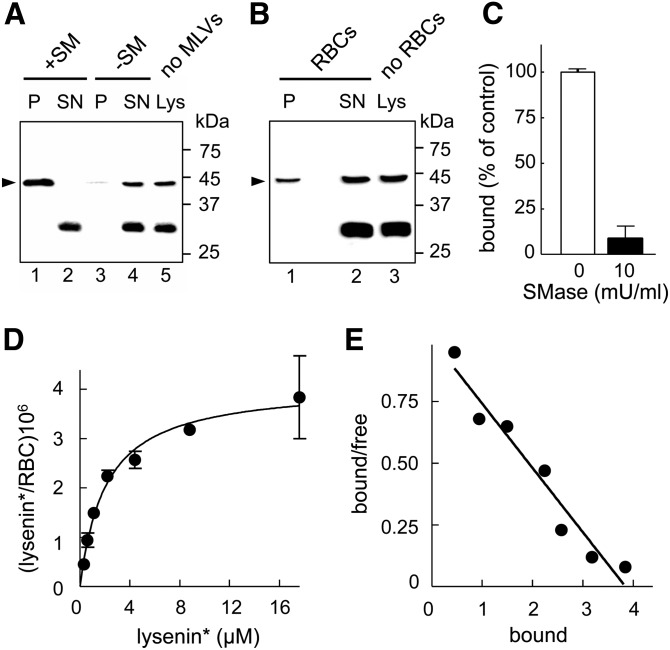
Fig. 1.
Specificity and quantification of lysenin* binding to endogenous SM in liposomes and RBCs in suspension. A, B: Specific binding of full lysenin*, but not its fragment at ∼30 kDa, to MLVs containing SM and to RBCs. A: Lysenin* was incubated or not (lane 5) with MLVs (1:5,000 molar ratio by reference to total lipids) containing SM (+SM; lanes 1 and 2) or not (−SM; lanes 3 and 4). B: Lysenin* was incubated or not (lane 3) in suspension with freshly isolated RBCs (∼1:20 molar ratio). After centrifugation, pellets (P) containing MLVs (A) or RBCs (B) and supernatants (SN) were analyzed by Western blotting for His-tag on lysenin*. Relative molecular masses (Mr) are indicated on the right. Arrowheads at left point to the expected position of full lysenin* (∼45 kDa). Pelleting of lysenin* depends on SM incorporation into liposomes. An N-terminal spurious fragment at ∼30 kDa was not detected in the pellet (P), neither after incubation with MLVs nor with RBCs. Representative blot from two independent experiments (A) and one experiment (B). C: Specific binding of 125I-lysenin* to SM in RBCs. Freshly isolated RBCs were either kept untreated (open bar) or depleted of SM by 10 mU/ml SMase (filled bar) prior to labeling in suspension with 125I-lysenin* (0.3 × 106 cpm) mixed with 1 μM cold lysenin* (in the continued presence of SMase at right). After washing by centrifugation/resuspension, counts per minute of 125I-lysenin* bound to RBCs were measured and expressed as percentage of control cells. SM depletion decreases lysenin* binding by ∼90%. D: Binding isotherm of 125I-lysenin* to RBCs. RBCs were incubated in suspension with 125I-lysenin* (0.3 × 106 cpm) together with the indicated concentrations of cold lysenin*. Bound lysenin* is expressed as (molecules/RBC) × 106 and is presented as the mean ± SEM of three samples. Representative of two independent experiments. E: Scatchard plot of data in (D). Bound values represent (molecules/RBC) × 106 and bound/free ratios are in nl/RBC. It has to be noticed that free values represent both the free “RBC-bindable” full lysenin* (∼45 kDa) and the “non-bindable” fragment (∼30 kDa).