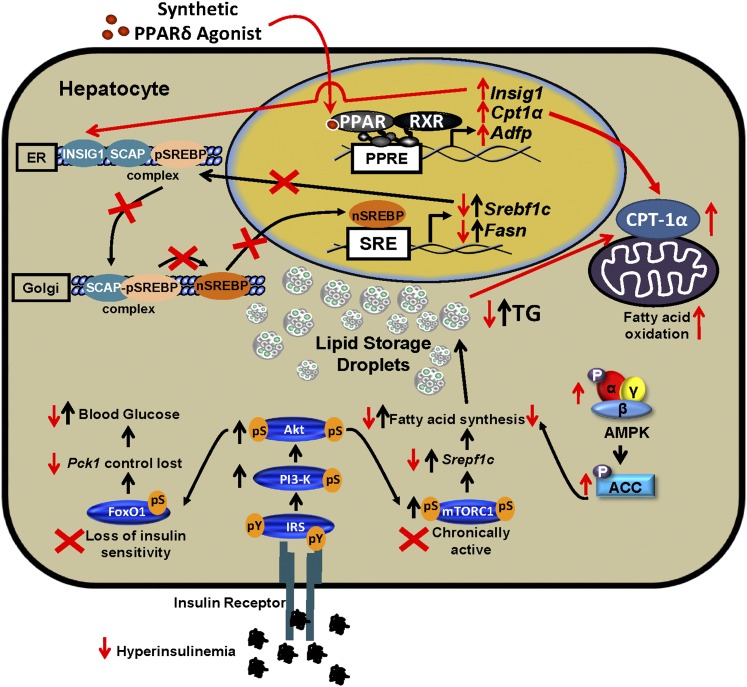
Fig. 7.
GW1516 intervention inhibits progression of hepatic steatosis in mice. Selective insulin resistance in the liver is induced in mice fed a high-fat diet. Despite hyperinsulinemia, insulin fails to decrease gluconeogenesis due to resistance of the FoxO1 pathway (loss of fasting/refeeding regulation of phospho-serine, pS), whereas the mTORC1/SREBP-1c pathway maintains insulin sensitivity (fasted pS-mTOR is elevated and increased even further with refeeding), leading to increased FA synthesis and TG accumulation. Pathways affected by diet are denoted by black arrows. Red arrows and crosses indicate the effects of GW1516 intervention. PPARδ activation attenuated hepatic TG accumulation as a consequence of increased FA oxidation and reduced FA synthesis. GW1516 stimulated FA oxidation due to increased Cpt1a expression. Increased hepatic AMPK activation by GW1516 was, in part, required for reduction of FA synthesis. The PPARδ agonist suppressed hyperinsulinemia, corrected signaling through the FoxO1 pathway, and normalized signaling through the mTORC1/SREBP-1c pathway. GW1516 decreased Srebf1c expression and inhibited the conversion of precursor SREBP-1c (pSREBP-1c) to the active nuclear form (nSREBP-1c), in part, due to increased expression of Insig1, which collectively contributed to the decreased FA synthesis. PPRE, PPAR response element; SCAP, SREBP cleavage-activating protein; SRE, sterol response element.