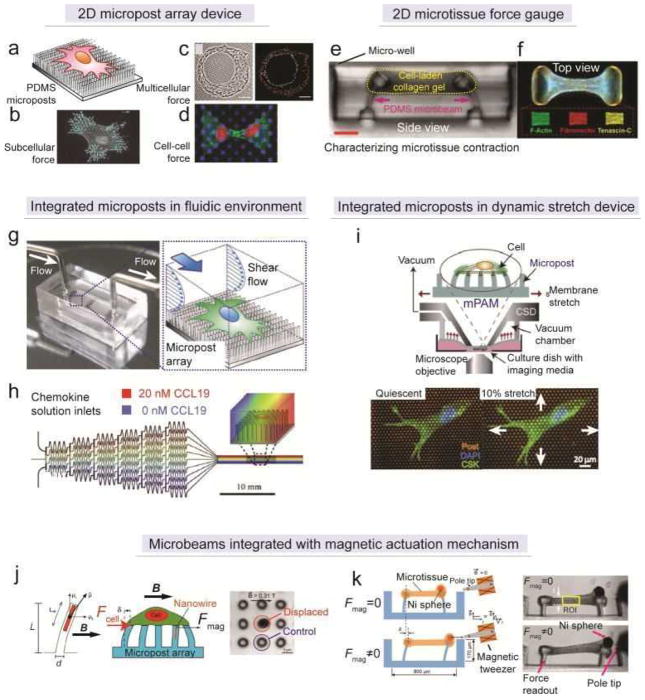
Figure 11. Quantification of cellular contractility by elastomeric microbeam bending.
(a) Schematic of the micropost array for monitoring cell-ECM contractile forces. (b) Processed fluorescent image showing subcellular cellular contractile forces within a single cell.[108] Reproduced with permission from [108]. Copyright 2011, Nature Publishing Group. (c) Phase contrast image (left) and processed traction force map (right) showing cell-ECM forces underneath a micro-patterned multi-cell cluster.[249] Reproduced with permission from [249]. Copyright 2007, United States National Academy of Sciences. (d) Fluorescent image showing the application of the micropost array in studying cell-cell tugging force.[227] Reproduced with permission from [227]. Copyright 2010, United States National Academy of Sciences. (e) Cross-section view of the microtissue force gauge (μTUG).[250] (f) Top view of the dog-bone like microtissue formed between two heads of microbeams after the gelation and contraction of cell-laden collagen gel.[250] Reproduced with permission from [250]. Copyright 2009, United States National Academy of Sciences. (g) Picture (left) and schematic (right) of a microfluidic channel with integrated micropost array for monitoring the dynamics of cell-ECM contractile forces during endothelial cell morphological remodeling under laminar shear flow.[253] Reproduced with permission from [253]. Copyright 2012, Royal Society of Chemistry. (h) Schematic of a microfluidic gradient generator with integrated micropost array for studying the dynamics of cell-ECM mechanical crosstalk during dendritic cells chemotaxis.[254] Reprodued with permission from [254]. Copyright 2011, Cell Press. (i) Schematic (upper) and fluorescent images (bottom) of a PDMS micropost array integrated with a cell-stretching device for studying the contractility of vascular smooth muscle cells under equibiaxial stretch.[255] Reproduced with permission from [255]. Copyright 2012, Royal Society of Chemistry. (j) Magnetic micropost array.[262] (left) Schematic of magnetic actuation using Co nanowire embedded in a PDMS micropost. (middle) Schematic of monitoring cell-ECM contractile forces during the magnetic actuation. (right) Phase contrast image of the bending of magnetic micropost under magnetic field. Reproduced with permission from [262]. Copyright 2007, United States National Academy of Science. (k) Magnetic microtissue force gauge.[264] (left panel) Schematic of magnetic actuation using Ni sphere incorporated within a microtissue. Either static or cyclic actuation could be achieved using the magnetic tweezers. (right panel) Cross-section view of the microtissue before and after magnetic actuation. The region of interest (ROI) was selected as the central part of the microtissue, and subsequent biomechanics analysis could be performed to extract the mechanical properties of the microtissue. Reproduced with permission from [264]. Copyright 2012, Wiley-VCH (Germany).