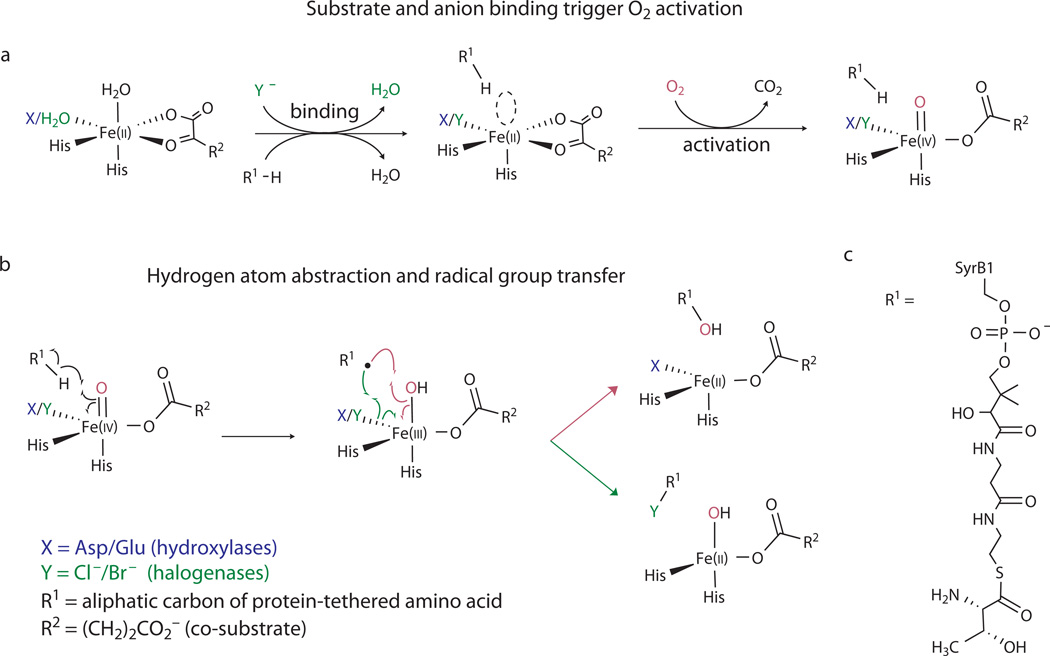
Figure 1. Related mechanistic logic of the Fe/αKG halogenases and hydroxylases.
(a) Steps leading to formation of the H•-abstracting (halo)ferryl complexes; (b) Divergence of the mechanisms of (halo)ferryl-mediated hydroxylation (top, magenta arrows) and halogenation (bottom, green arrows); (c) structure of the native SyrB2 substrate, SyrB1-appended L-threonine (Thr-S-SyrB1). The substrate used most extensively in this study had L-2-aminobutyric acid (Aba) appended to SyrB1 (Aba-S-SyrB1), which effectively replaced the side chain hydroxyl group of Thr-S-SyrB1 by hydrogen.