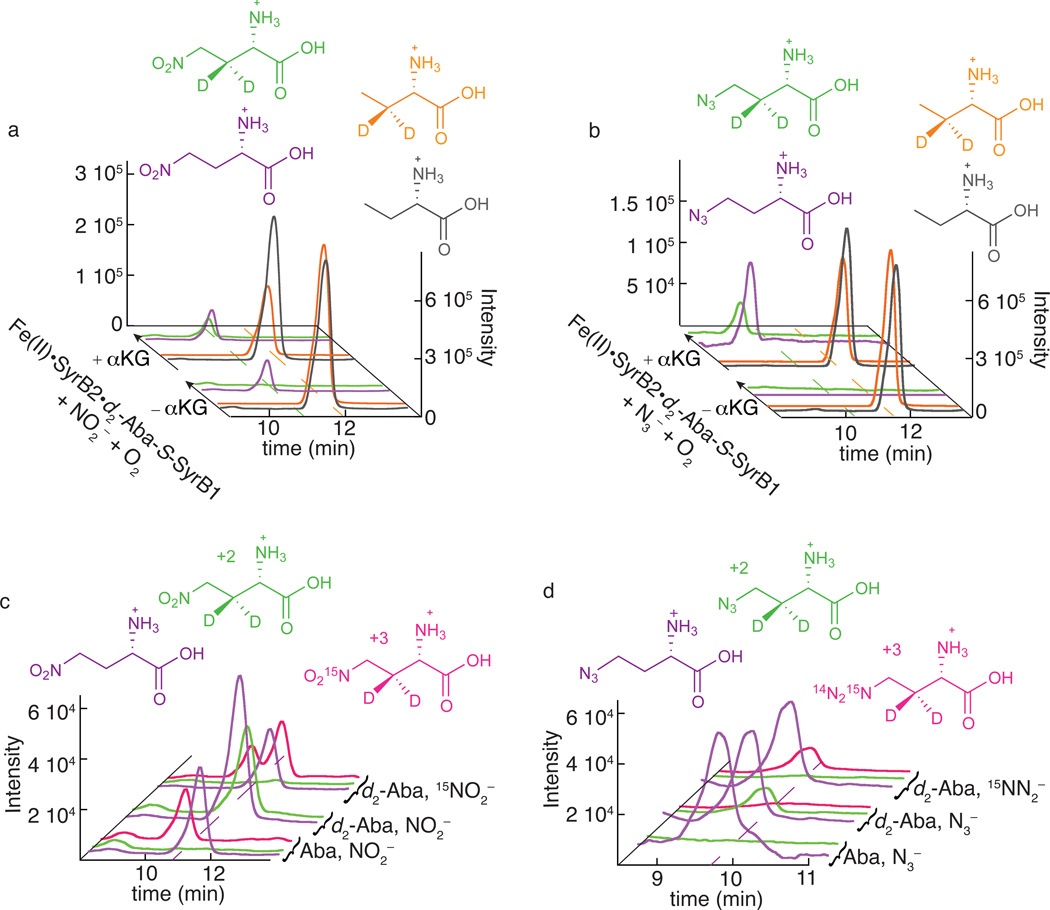
Figure 2. LC-MS analysis demonstrating the novel C-N coupling reactions catalyzed by SyrB2.
Panels a and c illustrate detection of the nitration product (1) and b and d the azidation product (2). In a and b, single-ion chromatograms at the m/z values for deuterium-labeled substrate (orange) and unlabeled standard (black) and for the deuterium-labeled enzymatic nitration/azidation product (green) and unlabeled standard (purple) are shown. The scaling applied to match the intensities of the peaks for the labeled Aba substrate and its unlabeled standard in the −αKG control reactions reveals consumption of the labeled substrate in the complete (+αKG) reaction by diminution of the orange peaks at back. The 4-NO2-3-d2-Aba (a, green) and 4-N3-3-d2-Aba (b, green) products co-eluting with the unlabeled synthetic standards (purple) are formed only in the presence of αKG. Note that the unlabeled 4-N3-Aba standard was omitted from the −αKG control sample (b, purple at front) to permit baseline correction of the corresponding chromatogram from the +αKG reaction sample. The single-ion chromatograms in c and d definitively show the attachment of the anions in the detected products by the elimination of the "+2" peaks for 4-14NO2/14N3-3-d2-Aba (green) and appearance of the "+3" peaks for 4-15NO2/15NN2-3-d2-Aba (magenta) upon use of the 15N-labeled anion. Reaction conditions, sample preparation, and LC-MS method are provided in Online Methods.