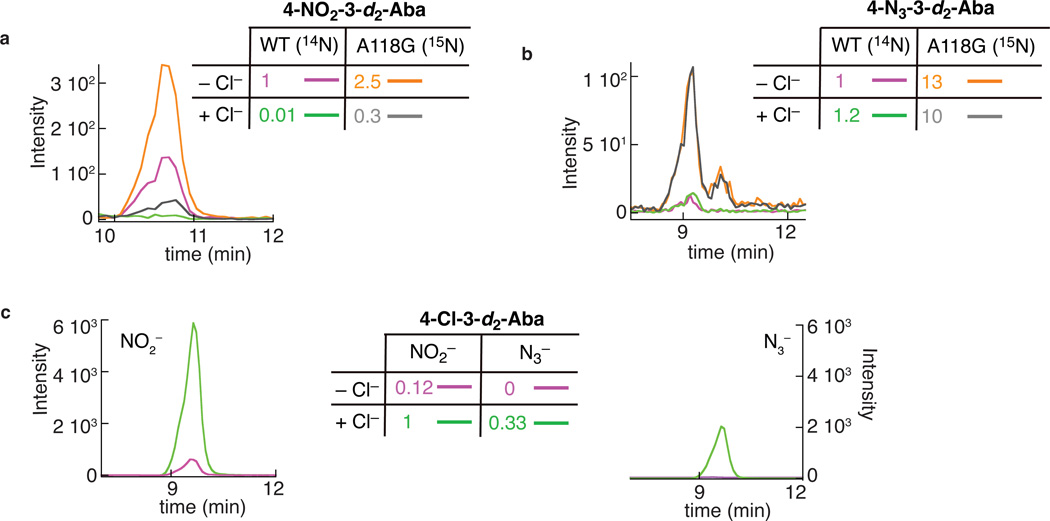
Figure 5. Enhanced efficiency of C–N coupling in the A118G variant of SyrB2.
The LC-MS/MS chromatograms monitor collision-induced transitions associated with the (a) nitration, (b) azidation, and (c) chlorination products formed in the reactions of the wild-type and A118G variant SyrB2. a and b: reactions of the wild-type protein in the presence of the natural-abundance anion and the A118G variant protein in the presence of 15NO2− or 15N14N2− were combined and co-injected. Purple and orange: reactions containing no added Cl−; green and gray: reactions containing 1 equiv per SyrB2(-A118G) added Cl−. Comparison of the orange and purple traces in a illustrates that the nitration yield of the A118G variant is ~ 2.5-fold greater than the yield of the wild-type enzyme in the absence of added Cl−; comparison of the gray and green traces implies a ~ 30-fold greater nitration yield for the A118G variant in the presence of one equivalent added Cl−. Cross comparisons of the orange and gray or purple and green traces illustrate the ability of added Cl− to suppress the nitration reaction in both wild-type SyrB2 and the A118G variant. Comparison of the orange and purple traces in b illustrates that the azidation yield of the A118G variant is ~ 13-fold greater than yield of the wild-type enzyme in the absence of added Cl−. Comparisons of the orange and gray or purple and green traces illustrate the inability of one equivalent added Cl− to suppress the azidation reaction in either wild-type or A118G variant SyrB2. c: chromatograms for the chlorination product showing that addition of Cl− (green) enhances the chlorination yields in both the reaction with NO2− (left) and the reaction with N3− (right) compared to the corresponding reactions without added Cl− (purple).