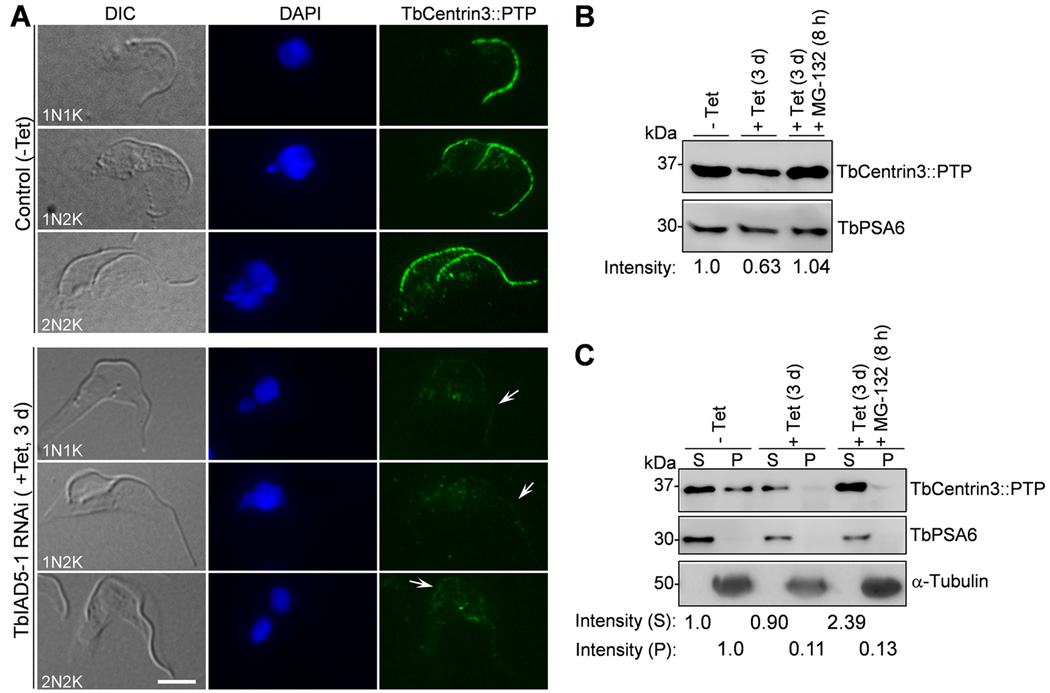
Figure 6. Effect of TbIAD5-1 knockdown on the localization and stability of TbCentrin3.
(A). TbIAD5-1 RNAi on TbCentrin3 localization to the flagellum. Endogenous TbCentrin3 was tagged with a C-terminal PTP epitope in cells harboring the TbIAD5-1 RNAi construct. RNAi was induced by tetracycline for 3 days, and cytoskeleton was prepared for immunostaining with anti-Protein A pAb and FITC-conjugated anti-rabbit IgG. Arrows indicate the weak TbCentrin3::PTP signal at the flagellum upon TbIAD5-1 RNAi. Bar: 5 µm. (B). Effect of TbIAD5-1 RNAi on TbCentrin3 protein stability. Crude lysate was analyzed by western blotting with anti-Protein A pAb to detect PTP-tagged TbCentrin3. Level of TbPSA6 served as the loading control. The intensity of protein bands was determined with ImageJ, and TbCentrin3 level was normalized with the loading control. (C). Effect of TbIAD5-1 RNAi on TbCentrin3 protein stability in cytosolic and the cytoskeletal fractions. Preparation of cytosolic and cytoskeletal fractions was carried out essentially as described in Fig. 5. TbCentrin3::PTP was detected by anti-Protein A pAb. α-Tubulin and TbPSA6 served as the control for cytoskeletal and cytosolic proteins, respectively. The intensity of protein bands was determined with ImageJ and normalized against the loading control. At least three repeats were performed.