FIG. 1.
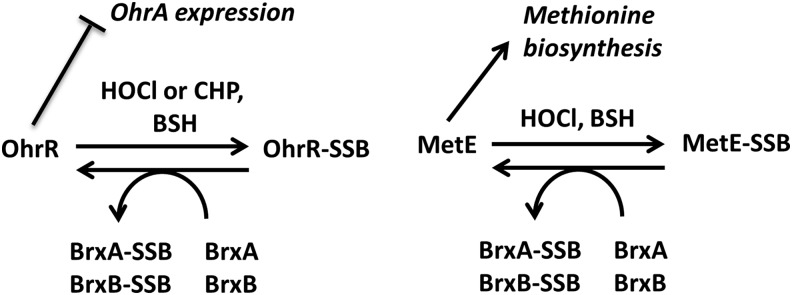
Model for S-bacillithiolation and de-bacillithiolation of OhrR and MetE indicating possible roles for the bacilliredoxins BrxA and BrxB. OhrR is a dimeric repressor that represses the transcription of ohrA (13). In response to oxidative stress, Bacillus subtilis OhrR is inactivated by S-bacillithiolation at its sole Cys residue (Cys15), leading to up-regulation of the OhrA peroxiredoxin that confers CHP and NaOCl resistance (4, 14). Oxidative stress also leads to S-bacillithiolation of the methionine synthase MetE at Cys730 and Cys719, resulting in methionine auxotrophy (4). The S-bacillithiolated OhrR-SSB and MetE-SSB proteins can be de-bacillithiolated in vitro by the BrxA and BrxB bacilliredoxins. Pathways for recycling of the Brx-SSB intermediate are not yet resolved. CHP, cumene hydroperoxide; NaOCl, sodium hypochlorite.
