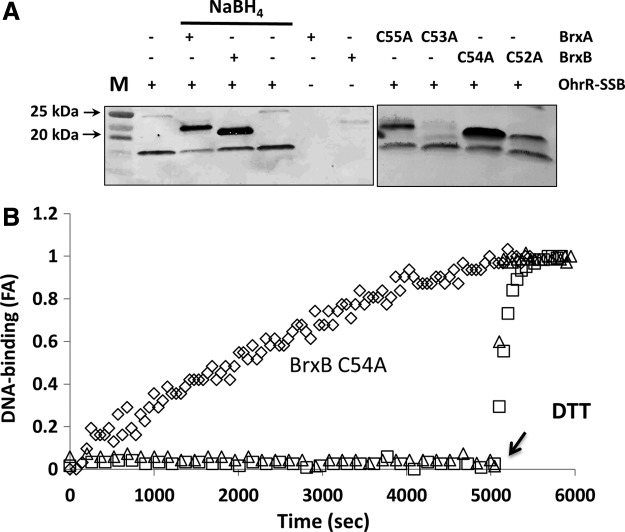
FIG. 4.
De-bacillithiolation of OhrR-SSB by BrxA and BrxB. (A) OhrR-SSB was generated in vitro using CHP and purified BSH, and the level of protein S-bacillithiolation was monitored by immunoblot analysis using anti-BSH antibodies. Left panel: Reduced BrxA and BrxB proteins (7.5 μM) (and a no protein control) were prepared by incubation with NaBH4 and tested for their ability to de-bacillithiolate OhrR (7.5 μM) as evidenced by a loss of OhrR-SSB signal and the appearance of S-bacillithiolated BrxA-SSB and BrxB-SSB. It should be noted that BrxA and BrxB are not detected unless they are S-bacillithiolated by incubation with OhrR-SSB. Right panel: Incubation of OhrR-SSB with BrxA C55A, BrxA C53A, BrxB C54A, or BrxB C52A indicates that thiol transfer occurs efficiently with those proteins which retain the active site (amino-terminal) Cys residue and inefficiently, if at all, with those retaining only the resolving Cys residue. (B) Ability of Brx proteins to restore DNA-binding activity of OhrR-SSB as monitored by FA analysis. Purified OhrR-SSB (300 nM) was incubated with fluorescently labeled ohrA operator DNA, and no binding (increased anisotropy) was observed. At time zero, various BrxA and BrxB proteins (3 μM) were added, and the regeneration of active (DNA-binding OhrR) was monitored over time. Under these conditions, BrxB C54A slowly reactivates OhrR-SSB (◊), but not S-cysteinylated OhrR (△). WT BrxA (not shown), BrxB (×), and BrxB C52A (□) were unable to reactivate OhrR.