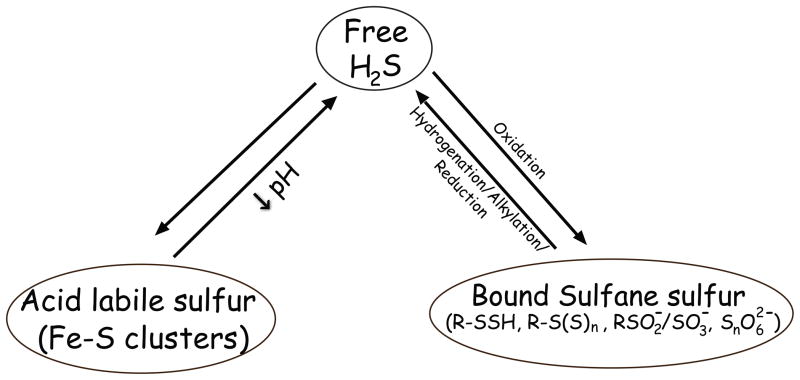
Figure 1. Various biochemical forms of H2S bioavailability.
H2S exists in different biochemical forms within biological systems that can be classified based on chemical properties and/or structure. Freely available H2S represents gaseous H2S and its HS− anion, acid labile sulfide represents iron-sulfur clusters and persulfides, and bound sulfane sulfur represents thiol sulfides, polysulfides, sulfate/sulfite, and bound elemental sulfur.