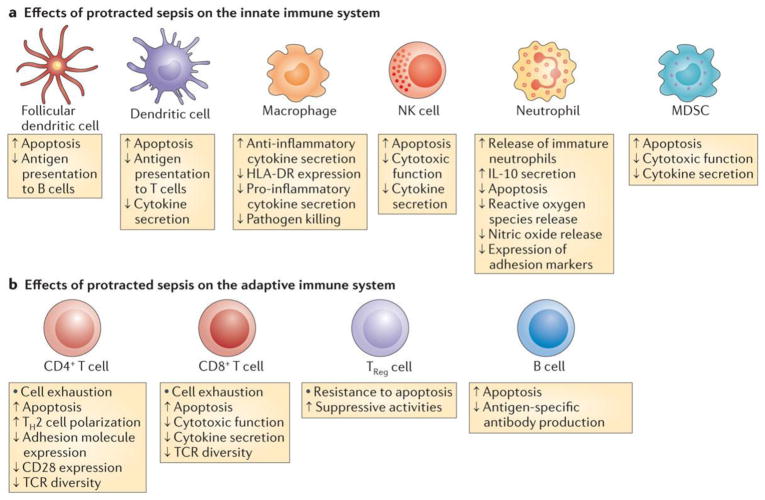
Figure 3. Impact of sepsis on innate and adaptive immunity.
a)Sepsis has diverse and profound effects on all cellular elements comprising the innate immune system. Sepsis rapidly triggers extensive apoptosis in dendritic cells, monocytes and immature macrophages, natural killer (NK) cells, and myeloid derived suppressor cells (MDSCs). Conversely, sepsis delays neutrophil apoptosis, a result thought to be secondary to the mechanisms of neutrophil activation. After initial mobilization and activation of neutrophils, subsequent neutrophils that are released from bone marrow have lower bactericidal functions and decreased cytokine production. Recent data show that a subset of neutrophils release large amounts of the immunosuppressive cytokine interleukin-10 (IL-10). Decreased HLA-DR expression on antigen presenting cells including monocyte/macrophages and dendritic cells is a hallmark of sepsis, which may impair the optimal presentation of microbial antigens to T cells. b) Sepsis causes massive loss of CD4+ and CD8+ T cells as well as B cells. T regulatory (Treg) cells are more resistant to sepsis-induced apoptosis and consequently, there is an increased percentage of TReg cells in the circulation relative to the other lymphocyte subsets. This contributes on a more immunosuppressive phenotype. Surviving CD4+ and CD8+ T cells have either a shift from a pro-inflammatory Th1 cell to an anti-inflammatory Th2 cell phenotype or develop an “exhaustive” phenotype characterized by increased programmed cell death-1 expression and reduced cytokine secretion. CD4+ T cells have decreased expression of CD28 and reduced T cell receptor (TCR) diversity, which both likely contributing to the impaired anti-microbial response to invading pathogens.