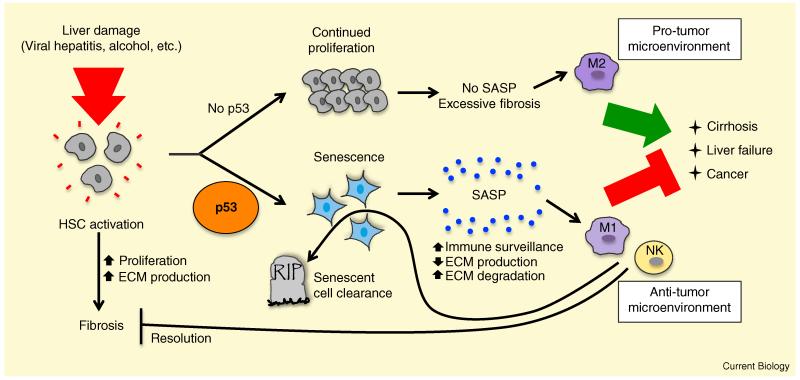
Figure 1.
p53 promotes senescence in hepatic stellate cells to maintain organ integrity and suppress cancer upon liver damage.
In response to liver damage, triggered by conditions such as viral hepatitis, alcohol abuse, and fatty liver disease, hepatic stellate cells (HSCs) become activated, proliferating and secreting extracellular matrix (ECM) components, leading to fibrosis. In the presence of p53 (bottom), HSCs undergo senescence, associated with the induction of the senescence-associated secretory phenotype (SASP). Secreted factors include those that regulate the extracellular matrix (ECM) and those that stimulate immune surveillance by polarizing macrophages to an anti-tumorigenic M1 phenotype and activating natural killer (NK) cells. Together, these responses inhibit cancer, trigger senescent cell clearance, and promote the resolution of fibrosis. In the absence of p53 activity (top), HSCs continue to proliferate and secrete ECM components, resulting in excessive fibrosis, cirrhosis, liver failure, and mortality. In addition, p53-deficient HSCs secrete factors that polarize macrophages to a pro-tumorigenic M2 fate, thereby promoting liver cancer.