Abstract
We analyzed T1-weighted brain magnetic resonance imaging data of 100 cognitively normal elderly controls (NC), 127 cognitively normal PD (PDCN), 31 PD-associated mild cognitive impairment (PDMCI) subjects from the Norwegian ParkWest study. Using automated segmentation methods, followed by the radial distance technique and multiple linear regression we studied the effect of clinical diagnosis on hippocampal and ventricular radial distance while adjusting for age, education and scanning site. PDCN subjects had significantly smaller bilateral hippocampal radial distance relative to NC. Nonamnestic PDMCI subjects showed smaller right hippocampal radial distance relative to cognitively normal elderly. PDMCI subjects showed significant enlargement of all portions of the lateral ventricles relative to cognitively NC and significantly larger bilateral temporal and occipital and left frontal lateral ventricular expansion relative to PDCN subjects. Nonamnestic PDMCI subjects showed significant ventricular enlargement spanning all parts of the lateral ventricle while those with amnestic PDMCI showed changes localized to the left occipital horn. Hippocampal atrophy and lateral ventricular enlargement show promise as structural biomarkers for PD.
Keywords: Parkinson disease, mild cognitive impairment, MCI, brain atrophy, hippocampal atrophy, ventricular enlargement, magnetic resonance imaging, MRI, cognitive correlations
1. Introduction
Cognitive impairment, one of the most understudied non-motor syndromes in Parkinson's disease (PD), is commonly seen even early in the disease course [Janvin, et al., 2006; Muslimovic, et al., 2005; Williams-Gray, et al., 2009] . PD subjects are at 2 to 6-fold increased risk for developing dementia relative to elderly controls [Breteler, et al., 1995] .
Structural MRI is an increasingly recognized platform for imaging biomarker development in neurodegeneration. Yet PD structural imaging biomarkers are significantly under developed. Hippocampal atrophy, the most established imaging biomarker for Alzheimer's disease (AD), is common to most forms of dementia [Apostolova and Thompson, 2007] , yet its presence in PD and PD dementia (PDD) has not been definitively established. While some groups have reported hippocampal atrophy in cognitively normal PD (PDCN) subjects [Camicioli, et al., 2003; Junque, et al., 2005] [Bruck, et al., 2004; Tam, et al., 2005] , others have not [Camicioli, et al., 2004] [Beyer, et al., 2007; Burton, et al., 2004; Kassubek, et al., 2002; Nagano-Saito, et al., 2005] . Several groups have also suggested that structural changes of the lateral ventricles occur in PD [Huber, et al., 1989; Meyer, et al., 2007] . The paper by Meyer et al [Meyer, et al., 2007] reported that subjects with MCI associated with presumed Lewy body pathology (i.e., PD or Dementia with Lewy bodies) showed greater enlargment of the third ventricle relative to MCI of the Alzheimer's disease type and vascular MCI, yet similar atrophy of the medial temporal lobe structures.
Recently, we published a study investigating the hippocampal and ventricular structural changes of PD-associated mild cognitive impairment (PDMCI, disease duration 10.5 years) and PDD subjects (disease duration 13.1 years) compared to PDCN (disease duration 14.3 years) and cognitively normal elderly (NC) [Apostolova, et al., 2010a] . While similar to others [Camicioli, et al.] we found significant ventricular enlargement of the lateral ventricles in PDD we were unable to show significant ventricular enlargement or hippocampal atrophy in PDMCI likely due to sample size restrictions. The goal of the present study was to investigate if ventricular enlargement is present in newly diagnosed drug-naïve PDMCI relative to newly diagnosed drug-naïve PDCN. With the larger ParkWest sample size our goal was also to more definitively address our hypothesis that PDMCI is associated with hippocampal atrophy. As PD is frequently unilateral at disease onset we repeated the regression analyses with the left- and right-predominant PD subjects only. We also investigated the associations between hippocampal and ventricular radial distance and global cognitive (MMSE) and disease severity indices (Unified Parkinson's Disease Rating Scale (UPDRS) and the modified Hoehn and Yahr (H&Y) staging).
2. Methods
2.1. Subjects
We analyzed the baseline structural magnetic resonance imaging (MRI) data from the Norwegian ParkWest study [Alves, et al., 2009] . ParkWest is a population-based multi-center prospective longitudinal study of newly diagnosed drug-naïve PD subjects aiming to define the clinical progression of PD over 10 years and to identify promising biomarkers for PDMCI and PDD. The protocol was reviewed and approved by the Regional Committee for Medical Research Ethics, University of Bergen, Norway. ParkWest recruitment strategies and diagnostic procedures have been described recently [Alves, et al., 2009] . Five neurology groups from Southwestern Norway actively participated in recruitment and research evaluations of enrolled participants. Study recruitment materials were sent to all hospital departments and general practitioner offices in Southwestern Norway. Reminders were sent twice during the study recruitment period. This comprehensive surveillance/referral mechanism resulted in 604 referrals of patients with possible PD between November 1 2004 and August 31 2006. All subjects were evaluated by one of the five participating neurology groups. Only newly diagnosed drug-naïve PD patients according to the Gelb diagnostic criteria for PD [Gelb, et al., 1999] were eligible for participation. ParkWest employs a multi-step diagnostic procedure. Screening and baseline assessments resulted in a provisional diagnosis. Subjects then received neurological follow-up once every 6 months and at each visit the provisional diagnosis of PD was carefully reappraised. The final ParkWest study diagnosis was made on an individual basis approximately 28 months after initial enrollment according to the Gelb criteria for PD [Gelb, et al., 1999] . The information used for the final study diagnosis included complete medical information from the screening and baseline research visits, as well as from the biannual clinical assessments. This included documentation of response to dopaminergic therapy and results from MRI and [123I] FP-CIT imaging when available. PD subjects were excluded from participation at any time during follow-up if they met criteria for the following parkinsonian disorders: Dementia with Lewy Bodies (DLB) according to the revised McKeith's criteria [McKeith, et al., 2005] , Multiple System Atrophy (MSA) according to the MSA consensus criteria [Gilman, et al., 1998] , Progressive Supranuclear Palsy (PSP) according to the National Institute of Neurological Disorders and the Society for PSP clinical research criteria [Litvan, et al., 1996] , monosymptomatic resting tremor based on the Consensus Statement of the Movement Disorder Society on Tremor [Deuschl, et al., 1998] . Subjects were also excluded if they had a history of strokes and/or stepwise progression of parkinsonism or had neuroradiologic findings of sufficient severity to be compatible with a diagnosis of “vascular parkinsonism”. Others were excluded if they had parkinsonian features that developed post exposure to neuroleptics or other drugs with antagonistic properties to dopamine receptors (i.e., a diagnosis of “drug-induced parkinsonism”), or received a diagnosis of essential tremor in patients presenting with predominantly postural upper limbs or head tremor of moderate amplitude with no other signs of parkinsonism which was not caused by medication, alcohol or hyperthyroidism.
ParkWest subjects are subjected to standardized clinical, neuropsychiatric and neuropsychological examinations, and brain MRI. As the goal of our study was to establish structural biomarkers for PDMCI, subjects who met criteria for dementia of any cause were excluded from our analyses as previously described [Alves, et al., 2009] . Severity of parkinsonian symptoms was assessed by the study physicians with the Unified Parkinson's Disease Rating Scale (UPDRS) [Fahn and Elton, 1987] and the modified Hoehn and Yahr (H&Y) staging [Hoehn and Yahr, 1967] . The neurospsychological test battery [Aarsland, et al., 2009] consisted of the Mini-Mental State Examination scale (MMSE) [Folstein, et al., 1975] , verbal memory assessment with the California Verbal Learning Test II (CVLT-2) [Delis, et al., 1987] , assessment of visuospatial abilities with the Silhouettes and Cube subtests from the Visual Object and Space Perception Battery (VOSP) [Warrington and James, 1991] , and assessment of attention and executive functions with the semantic verbal fluency/animal naming task [Benton and Hamsher, 1989] , the Stroop Test [Stroop, 1935] and the serial 7 test from the MMSE.
Raw cognitive scores for all PD subjects were converted to z-scores using the mean and standard deviations of the ParkWest age-matched NC group consisting of 205 individuals without parkinsonian symptoms, previous or current treatment with antiparkinsonian medication and any disease or symptom that could preclude completion of the study - including severe physical disability. PD subjects were classified as having PDMCI if their cognitive performance on at least one cognitive domain (memory, visuospatial and attention-executive) was more than 1.5 standard deviations below adjusted for age and education NC scores yet they were independent in their activities of daily living (i.e., did not meet criteria for dementia) [Aarsland, et al., 2009] . Impairment in the memory domain led to diagnosis of amnestic PDMCI, while preservation of memory with compromised non-memory cognitive function led to diagnosis of nonamnestic PDMCI. We note that there are currently no validated measures for soliciting cognitive complaints from patients with PD. Accordingly, indication of cognitive decline based on self or informant report, assessed by means of the IQCode and the UPDRS item 1, was considered supportive of a diagnosis of MCI but was not required.
Of the 604 PD screens conducted between November 1 2004 and August 31 2006, 265 subjects met provisional diagnosis of incident PD. 207 drug-naïve incident PD subjects agreed to longitudinal participation. 182 PD and 108 normal control (NC) subjects agreed to and received MRI. Of these, 258 ParkWest study participants (100 NC, 127 PDCN, 11 amnestic and 20 nonamnestic PDMCI) had useable imaging data (i.e., no motion or significant intensity artifacts, strokes or other structural lesions). Table 1 provides the demographic comparisons between PD subjects included in our analyses (N=158) and ParkWest PD participants who either did not agree or had contraindications to MRI, or whose scans were of insufficient quality for image analyses (N=49). PD subjects from the MRI cohort were significantly younger and more educated relative to the PD subjects who lacked useable scans. The MRI cohort also had significantly lower UPDRS motor subscale scores and higher MMSE (less cognitively and motorically impaired).
Table 1.
Demographic and clinical comparisons between PD subjects included in the analyses vs. those who were not
| Variable | PD with MRI N= 158 | PD without MRI N=49 | p-value |
|---|---|---|---|
| Age, yr | 66.8 (9.3) | 71.3(8.3) | 0.002 |
| Sex, M:F | 93/65 | 30/19 | 0.087$ |
| Education, yr | 11.3 (3.4) | 10.0 (2.6) | 0.004 |
| ApoE4+, N (%) | 56 (37) | 12(25.5) | 0.14$ |
| PD side, R/L/both | 75/63/20 | 23/17/8 | 0.732$ |
| H&Y | 1.9 (0.6) | 2.1 (0.8) | 0.169# |
| UPDRS | 22.0 (10.5) | 26.3 (12.7) | 0.019 |
| MMSE | 28.0 (2.1) | 26.7 (3.1) | 0.005 |
Mann Whitney U test
Chi square test
2.2. MRI acquisition and preprocessing
MRI was performed at four of the five study sites. The following protocols were used:
- Stavanger: 1.5 T Phillips Intera (Best, The Netherlands) TR/TE 10.0/4.6 msec, flip angle 30 degrees, 1 mm slices with no gap, NEX 2, Matrix 256×256
- Haugesund: 1.5 T Phillips Intera (Best, The Netherlands) TR/TE 20.0/4.6, flip angle 30 degrees, 1 mm slice thickness with no gap, NEX 1, Matrix 256×256
- Bergen: 1.5 T Siemens Symphony (Erlangen, Germany) TR/TE 2130.0/3.9, flip angle 15 degrees, 1 mm slice thickness with no gap, NEX 1, Matrix 256×256
- Arendal: 1.0 T Philips Intera system (Best, The Netherlands) TR/TE 25/6.9, flip angle 30 degrees, 2 mm slice thickness with no gap, NEX 1, Matrix 256×256.
T2-weighted and fluid attenuated inversed recovery (FLAIR) sequences were collected to evaluate subjects for strokes, and/or structural lesions. Subjects with these findings or with structural changes that could produce parkinsonian symptoms were excluded from the longitudinal imaging data collection and imaging analyses. Also excluded from the imaging analyses were subjects with baseline scan artifacts or scans of insufficient quality.
The 3D T1-weighted MR images were subjected to intensity normalization [Shattuck, et al., 2001] and spatial normalization to the International Consortium for Brain Mapping (ICBM53) brain atlas using the Minctracc algorithm and 9-parameter (9P) transformation (3 translations, 3 rotations, 3 scales) [Collins, et al., 1994] as previously described [Apostolova, et al., 2007] . The aligned images were resampled in an isotropic space of 220 voxels along each axis (x, y, and z) resulting in a final voxel size of 1 mm3.
2.3. Hippocampal segmentation
The hippocampal formations (including hippocampus proper, dentate gyrus and subiculum) of a randomly selected ParkWest training data set were manually segmented on gapless coronal slices by one experienced rater (MKB) blinded to subjects' age, sex and diagnosis following a detailed well-established protocol. The traces were closely inspected for accuracy by a second experienced hippocampal rater (LGA). The training dataset consisted of 29 subjects with 12 subjects (4 NC, 4PDCN and 4 PDMCI) from each of the two large imaging centers (Stavanger and Bergen), 3 subjects (one from each diagnostic group) from Arendal and 2 subjects from Haugesund (1 PDCN and 1 PDMCI). The training sample composition was proportionate to the ratio of subject enrollments among the four imaging centers, to prevent as far as possible any potential center bias in the statistical sampling.
Next the hippocampi of the full dataset were segmented with AdaBoost - our automated machine-learning hippocampal segmentation algorithm, based on the adaptive boosting approach. The algorithm uses thousands of voxel-specific features, such as image gradients, local curvatures at image interfaces, gray or white matter classification, statistical information on the likely stereotaxic position of the hippocampus, etc, to develop statistical rules for labeling each voxel in each new image as belonging to the hippocampus or not, based on the feature information contained in the positive and negative voxels of a training dataset. The AdaBoost algorithm has been extensively validated [Morra, et al., 2008a; Morra, et al., 2009] and utilized [Apostolova, et al., 2010b; Morra, et al., 2008a; Morra, et al., 2008b] .
2.4. Ventricular segmentation
We employed a previously validated semi-automated ventricular segmentation approach [Chou, et al., 2008] . Briefly, a human rater (MKB) first traced the lateral ventricles of 4 subjects and these traces were then converted into 3D parametric ventricular mesh models, termed atlases, as in [Thompson, et al., 2004] . Using fluid registration techniques each atlas was separately warped to match and thereby extract the shape of the lateral ventricle of each new subject's scan. This step resulted in four lateral ventricle segmentations per subject that were then averaged to create one final ventricular model. Averaging four separate segmentations minimizes automated labeling errors that occur when only one atlas is used.
2.5. Radial distance mapping
After modeling the segmented hippocampi and lateral ventricles as 3D parametric surface meshes, we computed the medial core (a medial curve threading down the center of each structure) and the radial distance from the medial core to each surface point for each structure in each subject [Apostolova, et al., 2006a; Apostolova, et al., 2006b; Thompson, et al., 2004] . Radial distance provides an intuitive measure of the thickness of the structure from its core to each point on its boundary.
2.6. Statistical methods
One-way analysis of variance (ANOVA) with post-hoc Bonferroni correction for multiple comparisons and chi-squared test were used to test for between-group differences in age, sex, education and the presence of Apolipoprotein E4 (ApoE4) genotype. The presence of ApoE4 allele was coded as 1, the absence was coded as 0. Subjects for whom ApoE4 genotype data was not available (6 NC, 9 PDCN and 3 PDMCI representing 6%, 7% and 10% of each group, respectively) were coded as 0.5. Due to non-normality of distribution of the MMSE scores, between-group comparisons were conducted with the Kruskal-Wallis test when three groups of comparison were compared (i.e., NC, PDNC and PDMCI) and Mann-Whitney when two comparison groups were compared (i.e., amnestic vs. nonamnestic PDMCI). We compared disease severity measured with the H&Y scale and the UPDRS motor subscale between PDCN and PDMCI, using a two-tailed Student's t-test.
Our main analyses were conducted with linear regression. The models included hippocampal and ventricular radial distance as the outcome and diagnosis as the predictor variable while adjusting for age, education, ApoE4 genotype and scanning site. Linear regression models with MMSE, UPDRS motor subscale and H&Y scores as predictors adjusting for center, and in the case of MMSE for ApoE4, were also performed in the pooled dataset. As PD is frequently unilateral at disease onset we repeated the regression analyses comparing left- and right-predominant PDCN and PDMCI subjects to the NC group. Cases with bilateral parkinsonian features were excluded from these models.
For map-wise multiple comparison correction we ran 100,000 permutations to measure the extent of the map that would have appeared significant by pure chance, in statistical maps thresholded at p<0.01[Thompson, et al., 2003] . The final permutation corrected p-value reflects the likelihood with which the observed experimental findings would have occurred by chance alone, in null data.
3. Results
Demographic and cognitive between-group comparisons are shown in Table 2 and 3, respectively. Comparing NC, PDCN and PDMCI revealed significant between-group differences in age, education and MMSE. After applying Bonferroni correction for multiple comparisons, we determined that the age difference was due to the PDMCI group being older than both the NC (p=0.012) and the PDCN groups (p=0.036). The difference in education was driven by a difference between the NC and PDCN groups (p=0.014). The groups were well balanced in respect to sex, disease lateralization, ApoE4 genotype, UPDRS and H&Y scores (Table 2). There were significant between-group cognitive differences on all cognitive measures with the PDMCI and PDCN groups performing significantly worse than our NC group (Table 3).
Table 2.
Demographic and clinical comparisons of the NC, PDCN and PDMCI groups (top) and the amnestic and nonamnestic PDMCI groups (bottom). Significant p-values in bold show group differences.
| Variable | NC N=100 | PDCN N=127 | PDMCI N=31 | p-value |
|---|---|---|---|---|
| Age, yr | 65.0 (9.4) | 65.8 (9.3) | 70.5 (8.1) | 0.015 |
| Sex, M:F | 48:52 | 74:53 | 19:12 | 0.2 |
| Education, yr | 12.6 (3.8) | 11.3 (3.4) | 11.6 (3.7) | 0.016 |
| ApoE4+, N (%) | 32 (32%) | 48 (38%) | 7 (23%) | 0.6 |
| PD side, R/L/both | N/A | 61/53/13 | 14/10/7 | 0.17 |
| H&Y | N/A | 1.8 (0.6) | 2.0 (0.5) | 0.2 |
| UPDRS | N/A | 21.7 (10.5) | 23.5 (10.4) | 0.4 |
| MMSE | 28.8 (1.2) | 28.3 (1.7) | 26.8 (3.0) | 0.001 |
| Variable | amnestic PDMCI N=11 | nonamnestic PDMCI N=20 | p-value | |
|---|---|---|---|---|
| Age, yr | 70.4 (6.0) | 70.6 (9.2) | 0.96 | |
| Sex (M:F) | 5:6 | 14:6 | 0.2 | |
| Education, yr | 11.5 (4.7) | 11.6 (3.2) | 0.96 | |
| ApoE4+, N (%) | 3 (27%) | 4 (20%) | 0.4 | |
| PD side, R/L/both | 4/5/2 | 10/5/5 | 0.5 | |
| H&Y | 2.3 (0.5) | 1.9 (0.4) | 0.05 | |
| UPDRS | 22.5 (13.1) | 24.0 (8.9) | 0.7 | |
| MMSE | 27.5 (2.1) | 26.5 (3.4) | 0.6 |
Table 3.
Cognitive comparisons of the NC, PDCN and PDMCI groups (top) and the amnestic and nonamnestic PDMCI groups (bottom). Unless otherwise marked we used Student's t-test or ANOVA with post-hoc Scheffe's correction for multiple comparisons. Significant p-values in bold show group differences.
| Variable | NC N=100 | PDCN N=127 | PDMCI N=31 | p-value |
|---|---|---|---|---|
| CVLT immediate recall | 8.9 (3.3) | 7.7 (3.2) | 4.3 (3.7) | <0.001 |
| CVLT total sumscore | 42.2 (11.3) | 38.7 (11.1) | 25.1 (11) | <0.001 |
| VOSP silhouette | 19.8 (3.5) | 19.7 (3.9) | 15.9 (4.5) | <0.001 |
| VOSP cube | 9.8 (0.5) | 9.5 (1.1) | 8.6 (1.7) | <0.001 |
| Serial 7's | 4.4 (0.92) | 4.2 (1.3) | 3.0 (2) | 0.001 |
| Stroop interference | 30.9 (8.3) | 29.3 (10.8) | 18.5 (9.2) | <0.001 |
| Sum of words from the Stroop test | 84.1 (13.0) | 80.2 (18.3) | 63.9 (19.1) | <0.001 |
| Semantic fluency | 19.1 (4.9) | 19.0 (5.2) | 14.5 (5.4) | <0.001 |
| Variable | amnestic PDMCI N=11 | nonamnestic PDMCI N=20 | p-value | |
|---|---|---|---|---|
| CVLT immediate recall | 1.8 (2.4) | 5.7 (3.6) | 0.003 | |
| CVLT total sumscore | 17 (7.3) | 30.1 (9.9) | 0.001 | |
| VOSP silhouette | 17.5 (4.8) | 15 (4.2) | 0.137 | |
| VOSP cube | 8.1 (2) | 9.0 (1.5) | 0.261# | |
| Serial 7's | 2.7 (2) | 3.2 (2) | 0.502# | |
| Stroop interference | 19.1 (8.3) | 17.7 (9.8) | 0.704 | |
| Sum of words from the Stroop test | 72.4 (17.9) | 58.9 (17.4) | 0.05 | |
| Semantic fluency | 15.4 (6.3) | 14.1 (5.1) | 0.545 |
Mann Whitney U test
Amnestic and nonamnestic PDMCI subjects showed comparable age, sex, educational level, disease lateralization, ApoE4 genotype, UPDRS motor subscale and MMSE distribution (Table 2). The only difference seen was in H&Y scores - amnestic PDMCI subjects showed borderline significant higher scores (p=0.05). While amnestic and nonamnestic PDMCI subjects performed comparably on VOSP, serial 7, Stroop interference and semantic fluency, amnestic MCI subjects showed significantly lower scores on CVLT and sum of words from the Stroop test (Table 3).
3.1. Hippocampal analyses
The 3D age-, education-, center− and ApoE4-adjusted hippocampal significance and percent difference maps for the between-group comparisons can be seen in Figure 1.
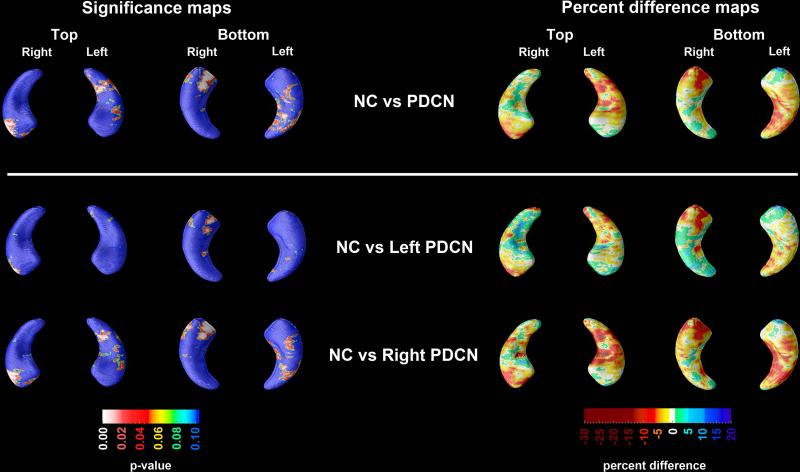
Figure 1.
3D significance (left panel) and percent difference (right panel) NC vs. PDCN hippocampal maps. Red and white areas in the significance maps show statistical significance (p<0.05). The following maps survived stringent correction for multiple comparisons: NC vs. PDCN on the right and NC vs. right-predominant PDCN vs. NC on the right.
PDCN vs. NC comparisons
The PDCN vs. NC 3D hippocampal radial distance statistical and percent difference maps are presented in Figure 1. Compared to NC, the PDCN group had significantly smaller right hippocampal radial distances (left pcorrected=0.061, right pcorrected=0.0086). Quantitatively, there was a 10-20% difference in radial distance in the areas of significance between the two groups (Figure 1). In the regression analyses using left- or right-predominant PDCN cases only (Figure 1) we found significant differences in right hippocampal radial distance between the right-predominant PDCN and NC (pcorrected=0.0077). Left-predominant PDCN subjects did not show significant differences relative to NC. ApoE4 was not a significant predictor of hippocampal radial distance in any of the regression models.
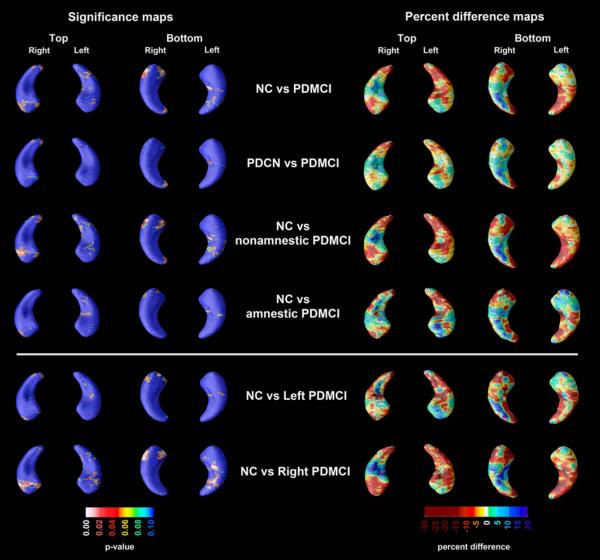
PDMCI vs. NC and PDCN comparisons
The PDMCI vs. NC and PDMCI vs. PDCN 3D hippocampal radial distance statistical and percent difference maps are presented in Figure 2. While some areas in both hippocampi showed up to 30% smaller radial distance in PDMCI relative to NC, these differences did not survive our stringent permutation correction for multiple comparisons. The differences in hippocampal radial distance between PDMCI and PDCN were of smaller magnitude than those seen between NC and PDMCI, and likewise did not reach statistical significance. Of the PDMCI subtypes, only the nonamnestic PDMCI group showed trend-level smaller right hippocampal radial distance relative to NC subjects (pcorrected=0.092). No trend-level or significant differences were seen between the amnestic PDMCI group and NC. Direct comparison of amnestic and nonamnestic PDMCI did not reveal significant differences in hippocampal radial distance (maps not shown). In the regression analyses using left- or right-predominant PDMCI cases only we found significant differences in right hippocampal radial distance between the right-predominant PDMCI and NC (p=0.017). Left-predominant PDMCI subjects did not show significant differences relative to NC. ApoE4 was not a significant predictor of hippocampal radial distance in any of the regression models.
Figure 2.
3D significance (left panel) and percent difference (right panel) NC vs. PDMCI hippocampal maps. Red and white areas in the significance maps show statistical significance (p<0.05). The NC vs. right-predominant PDMCI right hippocampal map survived stringent correction for multiple comparisons.
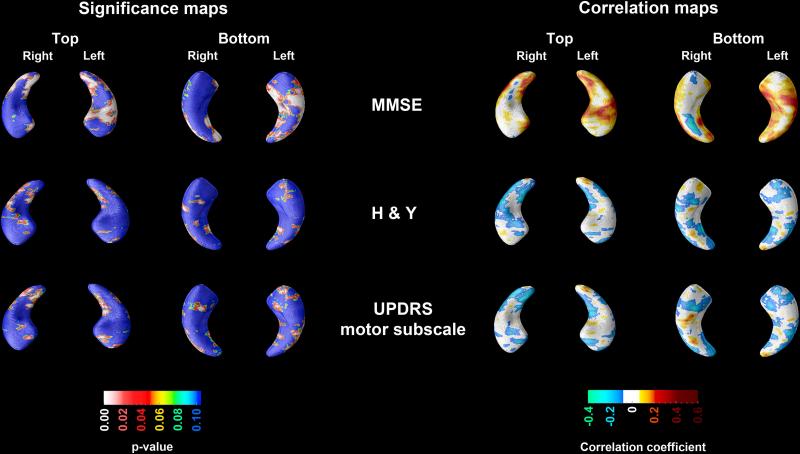
MMSE, H&Y and UPDRS associations
The center- and ApoE4-adjusted associations between hippocampal radial distance and MMSE, as well as the center-adjusted associations between hippocampal radial distance and H&Y and UPDRS motor subscale across the pooled sample can be seen in Figure 3. We found significant left (pcorrected<0.0001) and right (pcorrected=0.00035) positive associations with MMSE. UPDRS scores similarly showed negative associations with hippocampal radial distance bilaterally (left pcorrected=0.048; right pcorrected=0.036), while a trend-level negative effect for H&Y was present on the right only (pcorrected=0.06).
Figure 3.
3D significance (left panel) and correlation (right panel) maps of the association between MMSE, H&Y and UPDRS motor subscale scores and hippocampal radial distance. Areas in red and white in the significance maps show statistical significance (p<0.05). Both the left and right hippocampal MMSE and UPDRS maps survived stringent correction for multiple comparisons.
3.2. Ventricular analyses
PDCN vs. NC comparisons
PDCN subjects showed no significant ventricular enlargement relative to NC.
PDMCI vs. NC and PDCN comparisons
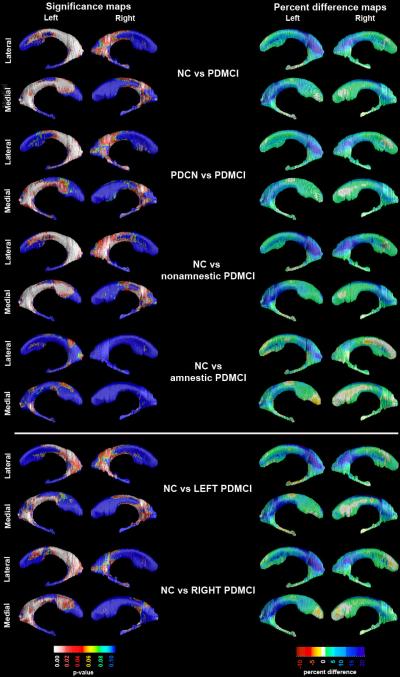
The PDMCI vs. NC and PDMCI vs. PDCN 3D hippocampal radial distance statistical and percent difference maps are presented in Figure 4. The PDMCI group showed greater left than right enlargement of the frontal, temporal and occipital lateral ventricular horns, relative to NC (left frontal pcorrected=0.0003, left temporal pcorrected=0.024, left occipital pcorrected=0.0003; left whole ventricle pcorrected=0.0082; right frontal pcorrected=0.036, right temporal pcorrected=0.08, right occipital pcorrected=0.036; right whole ventricle pcorrected=0.047). The magnitude of between-group differences in areas of significance ranged from 5 to 25%. Splitting the PDMCI sample into left- or right-predominant subgroups revealed significant contralateral frontal and occipital horn enlargement in right-predominant PDMCI (left frontal pcorrected=0.0044, left occipital pcorrected=0.0011; left whole ventricle pcorrected=0.07) and bilateral frontal and occipital differences in left-predominant PDMCI (left frontal pcorrected=0.0049, left occipital pcorrected=0.0058; left whole ventricle pcorrected=0.023; right frontal pcorrected=0.019, right occipital pcorrected=0.022; right whole ventricle pcorrected=0.034) relative to NC.
Figure 4.
3D significance (left panel) and percent difference (right panel) NC vs. PDMCI ventricular radial distance maps. Red and white areas in the significance maps show statistical significance (p<0.05). The following maps survived stringent correction for multiple comparisons: NC vs. PDMCI, PDCN vs. PDMCI, NC vs. nonamnestic PDMCI and NC vs. left-predominant PDMCI bilaterally, as well as NC vs. right-predominant PDMCI on the left.
Of the PDMCI subtypes, the nonamnestic PDMCI group showed extensive ventricular enlargement spanning all parts of the lateral ventricle relative to NC ranging from 5-25% (left frontal pcorrected=0.0006, left temporal pcorrected=0.03, left occipital pcorrected=0.0002; left whole ventricle pcorrected=0.01; right frontal pcorrected=0.016, right temporal pcorrected=0.056, right occipital pcorrected=0.016; right whole ventricle pcorrected=0.028) while amnestic PDMCI showed only a trend for ventricular enlargement of the left occipital horn relative to NC (pcorrected=0.0995, radial distance % difference range: 5-15%). Direct comparison of amnestic and nonamnestic PDMCI did not reveal significant differences in ventricular radial distance (maps not shown).
The PDMCI group showed larger bilateral temporal and occipital, and left frontal horn radial distance relative to PDCN (left frontal pcorrected=0.0088, left temporal pcorrected=0.022, left occipital pcorrected=0.0008; left whole ventricle pcorrected=0.01 right temporal pcorrected=0.0094, right occipital pcorrected=0.027; right whole ventricle pcorrected=0.048). In this comparison the magnitude of between-group differences was 5-20%. ApoE4 showed a trend-level association with the radial distance of the left body/occipital horn in the pooled sample only.
MMSE, H&Y and UPDRS associations
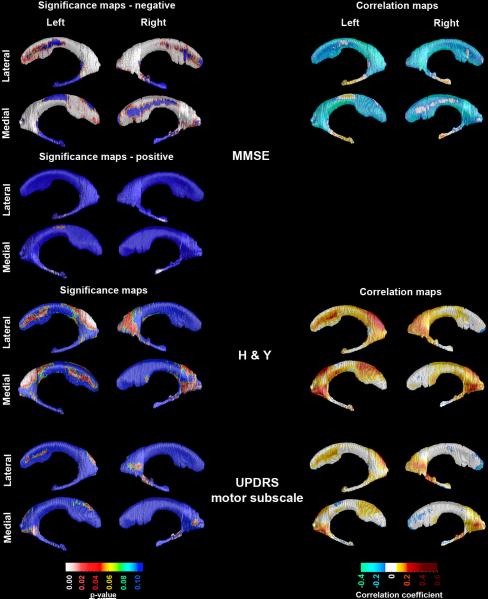
Figure 5 shows the center- and ApoE4-corrected significance and correlation maps between MMSE and ventricular radial distance, as well as the center-corrected significance and correlation maps between H&Y and UPDRS motor subscale scores and ventricular radial distance in the pooled sample. As expected there was a strong negative association between MMSE and the frontal (left pcorrected<0.0001; right pcorrected=0.0003) and body/occipital horns (left pcorrected<0.0001; right p=0.0001) of the lateral ventricles. For the temporal horns we observed a significant right (pcorrected=0.007) and trend-level left (pcorrected=0.06) positive association with MMSE (Figure 5 top panel). We also found significant associations between H&Y scores and the body/occipital horn on the left (left pcorrected=0.009) as well as trend-level right body/occipital horn (right pcorrected=0.06) and left frontal horn (left pcorrected=0.095, Figure 5 middle panel). The UPDRS motor subscale showed trend-level positive associations with left body/occipital (pcorrected=0.08) and right temporal horns (pcorrected=0.05, Figure 5 bottom panel).
Figure 5.
3D significance (left panel) and correlation (right panel) maps of the association between MMSE, H&Y and UPDRS motor subscale scores and ventricular radial distance. Areas in red and white in the significance maps show statistical significance (p<0.05). The left and right ventricular MMSE maps survived stringent correction for multiple comparisons.
4. Discussion
Hippocampal atrophy is the most established dementia biomarker to date and occurs in Alzheimer's disease [Apostolova, et al., 2010a; Apostolova, et al., 2006a; Apostolova, et al., 2006b; Jack, et al., 1997] , fronto-temporal [Kril and Halliday, 2004] and vascular dementias [Xu, et al., 2007] . Hippocampal atrophy in the MCI stage of AD is highly predictive of future development of dementia [Apostolova, et al., 2010a; Apostolova, et al., 2006b; Apostolova, et al., 2010c] . While most studies to date agree that hippocampal atrophy is present in the dementia stage of PD [Bouchard, et al., 2008; Camicioli, et al., 2003; Junque, et al., 2005; Tam, et al., 2005] , whether hippocampal atrophy is present in non-demented PD subjects has been controversial. Four studies – two using the region-of-interest (ROI) approach [Camicioli, et al., 2003; Junque, et al., 2005] and the other two a visual scale for hippocampal atrophy [Bruck, et al., 2004; Tam, et al., 2005] , reported hippocampal atrophy in PDCN, while one ROI [Camicioli, et al., 2004] and several voxel-based morphometry studies [Burton, et al., 2004; Kassubek, et al., 2002; Nagano-Saito, et al., 2005] failed to document hippocampal atrophy in PDCN.
Here we found that hippocampal atrophy and ventricular enlargement can occur early in the course of PD and are associated with cognitive decline. We detected hippocampal atrophy not only in the PDMCI state but also in our cognitively normal PD subjects. Hippocampal atrophy in PD was strongly associated with cognitive decline and disease severity as measured by the MMSE and the UPDRS motor subscale. Post mortem data [Bertrand, et al., 2004] and our current findings suggestive of hippocampal involvement as early as the PDCN stage indicate that more work in this area is warranted. It is important to establish whether PDCN subjects with hippocampal atrophy are at increased risk for cognitive decline. Further analyses of the longitudinal ParkWest dataset will allow us to address that question. Additionally, as the subset of PDMCI subjects grows we will be able to establish the prognostic significance of hippocampal atrophy in PDMCI.
Ventricular enlargement has likewise been observed in Alzheimer's disease [Carmichael, et al., 2007b; Chou, et al., 2010] , fronto-temporal [Kril and Halliday, 2004] , PDD [Apostolova, et al., 2010a] and vascular dementias [Carmichael, et al., 2007b] and has been reported to be predictive of cognitive decline to MCI and dementia [Carmichael, et al., 2007a; Chou, et al., 2010] . In our study we found pronounced ventricular enlargement in PDMCI but not in PDCN as we have previously reported [Dalaker, et al.] as well as a strong negative correlation between ventricular size and MMSE scores. Interestingly, nonamnestic PDMCI showed more pronounced ventriculomegaly relative to amnestic PDMCI subjects with greater involvement of the posterior and frontal parts of the lateral ventricles. As nonamnestic PDMCI subjects showed deficits in the visuospatial and attention-executive domains structural changes of the parietal and frontal lobes are to be expected. The question whether ventriculomegaly precedes or coincides with the onset of the PDMCI state will be addressed as we follow our PDCN cohort longitudinally. Taking into account the wealth of reports of cortical and subcortical atrophy in PDD [Apostolova, et al., 2010a; Beyer, et al., 2007; Burton, et al., 2005; Burton, et al., 2004; Nagano-Saito, et al., 2005; Ramirez-Ruiz, et al., 2005] and PDMCI [Beyer, et al., 2007; Meyer, et al., 2007] , one could speculate that the vetriculomegaly in PDMCI reflects cortical and subcortical involvement.
We consistently found left hemispheric involvement in our newly diagnosed drug-naïve PDMCI subjects regardless of disease laterality. Our disease lateralization analyses revealed significant contralateral ventricular and hippocampal predilection in right-predominant PDMCI subjects, but both contra- and ipsilateral ventricular enlargement in left-predominant PDMCI subjects relative to NC. One might argue that these findings potentially reflect a left-hemispheric bias in our neuropsychologic test battery, which included verbal as opposed to nonverbal memory and two verbal fluency tasks. However, if these right-predominant PDCN subjects convert to PDMCI and PDD at higher rates, our findings above would imply that right-sided PD poses subjects at greater risk for cognitive impairment, as has been previously suggested [Cooper, et al., 2009; Williams, et al., 2007] . We will be able to ascertain that once we analyze the longitudinal PDCN data. It is also worth mentioning that greater rates of lateral ventricle enlargement contralateral to the involved side has been reported by others and has been associated with faster decline in motor symptoms [Lewis, et al., 2009] .
Several strengths and limitations of the present study should be acknowledged. Major strengths include the excellent recruitment and disease ascertainment strategies employed in this population-based multi-center prospective longitudinal cohort study of drug naïve new onset PD. Advanced imaging methodology was also used for these analyses. At this time the major limitation of our analyses is that they are cross-sectional. However, as we proceed to analyses of 3-year and 5-year ParkWest structural MRI data that limitation will be overcome. Another limitation is the small sample size of the amnestic and nonamnestic PDMCI subgroups. This limitation is introduced by the study's strict inclusion criterion to enroll only newly diagnosed drug-naïve PD subjects. Such a design offers the opportunity to study PD along its longitudinal course since initial presentation, but limits our ability to have a large number of subjects meeting PDMCI criteria. Despite this limitation the nonamnestic PDMCI was sufficiently large to detect statistically significant differences. Finally, while correcting for scanning site reduces the variability introduced by the use of different scanners and different imaging protocols as much as possible, there is no doubt some residual unmodeled variance in our data, perhaps due to scanner effects. Yet despite this noise, we were able to find significant hippocampal and ventricular differences between the diagnostic groups. Had we analyzed a dataset with a unified imaging protocol and lesser scanner variability we might have had even better power to detect disease-associated differences.
Acknowledgements
The ParkWest study is funded by the Research Council of Norway [grant number 177966], the Western Norway Regional Health Authority [grant number 911218] and the Norwegian Parkinson's Disease Association.
Data analyses were supported by the National Institute of Aging [AG16570], the Easton Consortium for Alzheimer's Drug Discovery and Biomarker Development, the National Institute of Medical Imaging and Bioengineering [EB01651], the National Library of Medicine [LM05639] and the National Center for Research Resources [RR019771].
Footnotes
Disclosure Statement:
Liana Apostolova reports no conflicts of interest.
Guido Alves has received research support from GSK and the Norwegian Parkinson's disease Association, and honoraria for presentations from H. Lundbeck A/S and Orion Pharma.
Kristy S. Hwang reports no conflicts of interest.
Sona Babakchanian reports no conflicts of interest.
Kolbjørn Brønnick holds stocks in Dual Attention and has received honoraria for presentations from H. Lundbeck A/S and Solvay Pharma.
Jan Petter Larsen has served on scientific advisory boards for H. Lundbeck A/S and GSK.
Paul M. Thompson reports no conflicts of interest.
Yi-Yu Chou reports no conflicts of interest.
Ole Bjørn Tysnes has received honoraria for lectures and support to participate in scientific meetings from several companies engaged in Parkinson's disease.
Mona K. Beyer reports no conflicts of interest.
None of the author's institutions has contracts relating to this research through which it or any other organization may stand to gain financially now or in the future.
The protocol was reviewed and approved by the Regional Committee for Medical Research Ethics, University of Bergen, Norway.
References
- Aarsland D, Bronnick K, Larsen JP, Tysnes OB, Alves G. Cognitive impairment in incident, untreated Parkinson disease: the Norwegian ParkWest study. Neurology. 2009;72:1121–6. doi: 10.1212/01.wnl.0000338632.00552.cb. [DOI] [PubMed] [Google Scholar]
- Alves G, Muller B, Herlofson K, Hogenesch I, Telstad W, Aarsland D, Tysnes OB, Larsen JP. Incidence of Parkinson's disease in Norway. The Norwegian ParkWest study. J Neurol Neurosurg Psychiatry. 2009 doi: 10.1136/jnnp.2008.168211. [DOI] [PubMed] [Google Scholar]
- Apostolova LG, Beyer M, Green AE, Hwang KS, Morra JH, Chou YY, Avedissian C, Aarsland D, Janvin CC, Larsen JP, Cummings JL, Thompson PM. Hippocampal, caudate, and ventricular changes in Parkinson's disease with and without dementia. Mov Disord. 2010a;25:687–8. doi: 10.1002/mds.22799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apostolova LG, Dinov ID, Dutton RA, Hayashi KM, Toga AW, Cummings JL, Thompson PM. 3D comparison of hippocampal atrophy in amnestic mild cognitive impairment and Alzheimer's disease. Brain. 2006a;129:2867–73. doi: 10.1093/brain/awl274. [DOI] [PubMed] [Google Scholar]
- Apostolova LG, Dutton RA, Dinov ID, Hayashi K, Toga AW, Cummings JL, Thompson P. Conversion of mild cognitive impairment to Alzheimer disease predicted by hippocampal atrophy maps. Arch Neurol. 2006b;63:693–9. doi: 10.1001/archneur.63.5.693. [DOI] [PubMed] [Google Scholar]
- Apostolova LG, Morra JH, Green AE, Hwang KS, Avedissian C, Woo E, Cummings JL, Toga AW, Jack CR, Weiner MW, Thompson PM. Automated 3D mapping of baseline and 12-month associations between three verbal memory measures and hippocampal atrophy in 490 ADNI subjects. Neuroimage. 2010b doi: 10.1016/j.neuroimage.2009.12.125. submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apostolova LG, Steiner CA, Akopyan GG, Toga AW, Cummings JL, Thompson PM. 3D gray matter atrophy mapping in mild cognitive impairment and mild Alzheimer's disease. Arch Neurol. 2007;64:1489–95. doi: 10.1001/archneur.64.10.1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apostolova LG, Thompson PM. Brain mapping as a tool to study neurodegeneration. Neurotherapeutics. 2007;4:387–400. doi: 10.1016/j.nurt.2007.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apostolova LG, Thompson PM, Green AE, Hwang KS, Zoumalan C, Jack CR, Jr., Harvey DJ, Petersen RC, Thal LJ, Aisen PS, Toga AW, Cummings JL, Decarli CS. 3D comparison of low, intermediate, and advanced hippocampal atrophy in MCI. Hum Brain Mapp. 2010c;31:786–97. doi: 10.1002/hbm.20905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benton AL, Hamsher K. Multilingual apahasia examination. AJA Associates; Iowa City, Iowa: 1989. [Google Scholar]
- Bertrand E, Lechowicz W, Szpak GM, Lewandowska E, Dymecki J, Wierzba-Bobrowicz T. Limbic neuropathology in idiopathic Parkinson's disease with concomitant dementia. Folia Neuropathol. 2004;42:141–50. [PubMed] [Google Scholar]
- Beyer MK, Janvin CC, Larsen JP, Aarsland D. A magnetic resonance imaging study of patients with Parkinson's disease with mild cognitive impairment and dementia using voxel-based morphometry. J Neurol Neurosurg Psychiatry. 2007;78:254–9. doi: 10.1136/jnnp.2006.093849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouchard TP, Malykhin N, Martin WR, Hanstock CC, Emery DJ, Fisher NJ, Camicioli RM. Age and dementia-associated atrophy predominates in the hippocampal head and amygdala in Parkinson's disease. Neurobiol Aging. 2008;29:1027–39. doi: 10.1016/j.neurobiolaging.2007.02.002. [DOI] [PubMed] [Google Scholar]
- Breteler MM, de Groot RR, van Romunde LK, Hofman A. Risk of dementia in patients with Parkinson's disease, epilepsy, and severe head trauma: a register-based follow-up study. Am J Epidemiol. 1995;142:1300–5. doi: 10.1093/oxfordjournals.aje.a117597. [DOI] [PubMed] [Google Scholar]
- Bruck A, Kurki T, Kaasinen V, Vahlberg T, Rinne JO. Hippocampal and prefrontal atrophy in patients with early non-demented Parkinson's disease is related to cognitive impairment. J Neurol Neurosurg Psychiatry. 2004;75:1467–9. doi: 10.1136/jnnp.2003.031237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton EJ, McKeith IG, Burn DJ, O'Brien JT. Brain atrophy rates in Parkinson's disease with and without dementia using serial magnetic resonance imaging. Mov Disord. 2005;20:1571–6. doi: 10.1002/mds.20652. [DOI] [PubMed] [Google Scholar]
- Burton EJ, McKeith IG, Burn DJ, Williams ED, O'Brien JT. Cerebral atrophy in Parkinson's disease with and without dementia: a comparison with Alzheimer's disease, dementia with Lewy bodies and controls. Brain. 2004;127:791–800. doi: 10.1093/brain/awh088. [DOI] [PubMed] [Google Scholar]
- Camicioli R, Moore MM, Kinney A, Corbridge E, Glassberg K, Kaye JA. Parkinson's disease is associated with hippocampal atrophy. Mov Disord. 2003;18:784–90. doi: 10.1002/mds.10444. [DOI] [PubMed] [Google Scholar]
- Camicioli R, Sabino J, Gee M, Bouchard T, Fisher N, Hanstock C, Emery D, Martin WR. Ventricular dilatation and brain atrophy in patients with Parkinson's disease with incipient dementia. Mov Disord. doi: 10.1002/mds.23700. [DOI] [PubMed] [Google Scholar]
- Camicioli RM, Korzan JR, Foster SL, Fisher NJ, Emery DJ, Bastos AC, Hanstock CC. Posterior cingulate metabolic changes occur in Parkinson's disease patients without dementia. Neurosci Lett. 2004;354:177–80. doi: 10.1016/j.neulet.2003.09.076. [DOI] [PubMed] [Google Scholar]
- Carmichael OT, Kuller LH, Lopez OL, Thompson PM, Dutton RA, Lu A, Lee SE, Lee JY, Aizenstein HJ, Meltzer CC, Liu Y, Toga AW, Becker JT. Cerebral ventricular changes associated with transitions between normal cognitive function, mild cognitive impairment, and dementia. Alzheimer Dis Assoc Disord. 2007a;21:14–24. doi: 10.1097/WAD.0b013e318032d2b1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmichael OT, Kuller LH, Lopez OL, Thompson PM, Dutton RA, Lu A, Lee SE, Lee JY, Aizenstein HJ, Meltzer CC, Liu Y, Toga AW, Becker JT. Ventricular volume and dementia progression in the Cardiovascular Health Study. Neurobiol Aging. 2007b;28:389–97. doi: 10.1016/j.neurobiolaging.2006.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou YY, Lepore N, de Zubicaray GI, Carmichael OT, Becker JT, Toga AW, Thompson PM. Automated ventricular mapping with multi-atlas fluid image alignment reveals genetic effects in Alzheimer's disease. Neuroimage. 2008;40:615–30. doi: 10.1016/j.neuroimage.2007.11.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou YY, Lepore N, Saharan P, Madsen SK, Hua X, Jack CR, Shaw LM, Trojanowski JQ, Weiner MW, Toga AW, Thompson PM. Ventricular maps in 804 ADNI subjects: correlations with CSF biomarkers and clinical decline. Neurobiol Aging. 2010;31:1386–400. doi: 10.1016/j.neurobiolaging.2010.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins DL, Neelin P, Peters TM, Evans AC. Automatic 3D intersubject registration of MR volumetric data in standardized Talairach space. J Comput Assist Tomogr. 1994;18:192–205. [PubMed] [Google Scholar]
- Cooper CA, Mikos AE, Wood MF, Kirsch-Darrow L, Jacobson CE, Okun MS, Rodriguez RL, Bowers D, Fernandez HH. Does laterality of motor impairment tell us something about cognition in Parkinson disease? Parkinsonism Relat Disord. 2009;15:315–7. doi: 10.1016/j.parkreldis.2008.07.009. [DOI] [PubMed] [Google Scholar]
- Dalaker TO, Zivadinov R, Ramasamy DP, Beyer MK, Alves G, Bronnick KS, Tysnes OB, Aarsland D, Larsen JP. Ventricular enlargement and mild cognitive impairment in early Parkinson's disease. Mov Disord. 26:297–301. doi: 10.1002/mds.23443. [DOI] [PubMed] [Google Scholar]
- Delis DC, Kramer JH, Kaplan E, Ober BA. California Verbal Learning Test, Form II. 2nd ed. The Psychological Corporation; San Antonio, TX: 1987. [Google Scholar]
- Deuschl G, Bain P, Brin M. Consensus statement of the Movement Disorder Society on Tremor. Ad Hoc Scientific Committee. Mov Disord. 1998;13(Suppl 3):2–23. doi: 10.1002/mds.870131303. [DOI] [PubMed] [Google Scholar]
- Fahn S, Elton RL. Unified Parkinson's Disease Rating Scale. In: S Fahn, Marsden CD, Calne DM, Lieberman A., editors. Recent development in Parkinson's disease. MacMillan Health Care Information; Florham Park, NJ: 1987. pp. 153–63. [Google Scholar]
- Folstein M, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatric Res. 1975;12:189–98. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Gelb DJ, Oliver E, Gilman S. Diagnostic criteria for Parkinson disease. Arch Neurol. 1999;56:33–9. doi: 10.1001/archneur.56.1.33. [DOI] [PubMed] [Google Scholar]
- Gilman S, Low PA, Quinn N, Albanese A, Ben-Shlomo Y, Fowler CJ, Kaufmann H, Klockgether T, Lang AE, Lantos PL, Litvan I, Mathias CJ, Oliver E, Robertson D, Schatz I, Wenning GK. Consensus statement on the diagnosis of multiple system atrophy. Journal of the autonomic nervous system. 1998;74:189–92. [PubMed] [Google Scholar]
- Hoehn MM, Yahr MD. Parkinsonism: onset, progression and mortality. Neurology. 1967;17:427–42. doi: 10.1212/wnl.17.5.427. [DOI] [PubMed] [Google Scholar]
- Huber SJ, Shuttleworth EC, Christy JA, Chakeres DW, Curtin A, Paulson GW. Magnetic resonance imaging in dementia of Parkinson's disease. J Neurol Neurosurg Psychiatry. 1989;52:1221–7. doi: 10.1136/jnnp.52.11.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR, Jr., Petersen RC, Xu YC, Waring SC, O'Brien PC, Tangalos EG, Smith GE, Ivnik RJ, Kokmen E. Medial temporal atrophy on MRI in normal aging and very mild Alzheimer's disease.[see comment]. Neurology. 1997;49:786–94. doi: 10.1212/wnl.49.3.786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janvin CC, Larsen JP, Aarsland D, Hugdahl K. Subtypes of mild cognitive impairment in Parkinson's disease: progression to dementia. Mov Disord. 2006;21:1343–9. doi: 10.1002/mds.20974. [DOI] [PubMed] [Google Scholar]
- Junque C, Ramirez-Ruiz B, Tolosa E, Summerfield C, Marti MJ, Pastor P, Gomez-Anson B, Mercader JM. Amygdalar and hippocampal MRI volumetric reductions in Parkinson's disease with dementia. Mov Disord. 2005;20:540–4. doi: 10.1002/mds.20371. [DOI] [PubMed] [Google Scholar]
- Kassubek J, Juengling FD, Hellwig B, Spreer J, Lucking CH. Thalamic gray matter changes in unilateral Parkinsonian resting tremor: a voxel-based morphometric analysis of 3-dimensional magnetic resonance imaging. Neurosci Lett. 2002;323:29–32. doi: 10.1016/s0304-3940(02)00111-8. [DOI] [PubMed] [Google Scholar]
- Kril JJ, Halliday GM. Clinicopathological staging of frontotemporal dementia severity: correlation with regional atrophy. Dement Geriatr Cogn Disord. 2004;17:311–5. doi: 10.1159/000077161. [DOI] [PubMed] [Google Scholar]
- Lewis MM, Smith AB, Styner M, Gu H, Poole R, Zhu H, Li Y, Barbero X, Gouttard S, McKeown MJ, Mailman RB, Huang X. Asymmetrical lateral ventricular enlargement in Parkinson's disease. Eur J Neurol. 2009;16:475–81. doi: 10.1111/j.1468-1331.2008.02430.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litvan I, Agid Y, Calne D, Campbell G, Dubois B, Duvoisin RC, Goetz CG, Golbe LI, Grafman J, Growdon JH, Hallett M, Jankovic J, Quinn NP, Tolosa E, Zee DS. Clinical research criteria for the diagnosis of progressive supranuclear palsy (Steele-Richardson-Olszewski syndrome): report of the NINDS-SPSP international workshop. Neurology. 1996;47:1–9. doi: 10.1212/wnl.47.1.1. [DOI] [PubMed] [Google Scholar]
- McKeith IG, Dickson DW, Lowe J, Emre M, O'Brien JT, Feldman H, Cummings J, Duda JE, Lippa C, Perry EK, Aarsland D, Arai H, Ballard CG, Boeve B, Burn DJ, Costa D, Del Ser T, Dubois B, Galasko D, Gauthier S, Goetz CG, Gomez-Tortosa E, Halliday G, Hansen LA, Hardy J, Iwatsubo T, Kalaria RN, Kaufer D, Kenny RA, Korczyn A, Kosaka K, Lee VM, Lees A, Litvan I, Londos E, Lopez OL, Minoshima S, Mizuno Y, Molina JA, Mukaetova-Ladinska EB, Pasquier F, Perry RH, Schulz JB, Trojanowski JQ, Yamada M. Diagnosis and management of dementia with Lewy bodies: third report of the DLB Consortium. Neurology. 2005;65:1863–72. doi: 10.1212/01.wnl.0000187889.17253.b1. [DOI] [PubMed] [Google Scholar]
- Meyer JS, Huang J, Chowdhury MH. MRI confirms mild cognitive impairments prodromal for Alzheimer's, vascular and Parkinson-Lewy body dementias. J Neurol Sci. 2007;257:97–104. doi: 10.1016/j.jns.2007.01.016. [DOI] [PubMed] [Google Scholar]
- Morra JH, Tu Z, Apostolova LG, Green AE, Avedissian C, Madsen SK, Parikshak N, Hua X, Toga AW, Jack CR, Jr., Weiner MW, Thompson PM. Validation of a fully automated 3D hippocampal segmentation method using subjects with Alzheimer's disease mild cognitive impairment, and elderly controls. Neuroimage. 2008a;43:59–68. doi: 10.1016/j.neuroimage.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morra JH, Tu Z, Apostolova LG, Green AE, Avedissian C, Madsen SK, Parikshak N, Toga AW, Jack CR, Jr., Schuff N, Weiner MW, Thompson PM. Automated mapping of hippocampal atrophy in 1-year repeat MRI data from 490 subjects with Alzheimer's disease, mild cognitive impairment, and elderly controls. Neuroimage. 2008b doi: 10.1016/j.neuroimage.2008.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morra JH, Tu Z, Apostolova LG, Green AE, Toga AW, Thompson PM. Comparison of AdaBoost and support vector machines for detecting Alzheimer's disease through automated hippocampal segmentation. IEEE Trans Med Imaging. 2009;29:30–43. doi: 10.1109/TMI.2009.2021941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muslimovic D, Post B, Speelman JD, Schmand B. Cognitive profile of patients with newly diagnosed Parkinson disease. Neurology. 2005;65:1239–45. doi: 10.1212/01.wnl.0000180516.69442.95. [DOI] [PubMed] [Google Scholar]
- Nagano-Saito A, Washimi Y, Arahata Y, Kachi T, Lerch JP, Evans AC, Dagher A, Ito K. Cerebral atrophy and its relation to cognitive impairment in Parkinson disease. Neurology. 2005;64:224–9. doi: 10.1212/01.WNL.0000149510.41793.50. [DOI] [PubMed] [Google Scholar]
- Ramirez-Ruiz B, Marti MJ, Tolosa E, Bartres-Faz D, Summerfield C, Salgado-Pineda P, Gomez-Anson B, Junque C. Longitudinal evaluation of cerebral morphological changes in Parkinson's disease with and without dementia. J Neurol. 2005;252:1345–52. doi: 10.1007/s00415-005-0864-2. [DOI] [PubMed] [Google Scholar]
- Shattuck DW, Sandor-Leahy SR, Schaper KA, Rottenberg DA, Leahy RM. Magnetic resonance image tissue classification using a partial volume model. Neuroimage. 2001;13:856–76. doi: 10.1006/nimg.2000.0730. [DOI] [PubMed] [Google Scholar]
- Stroop JR. Studies in interference of serial verbal reactions. Journal of Experimental Psychology. 1935;18:643–62. [Google Scholar]
- Tam CW, Burton EJ, McKeith IG, Burn DJ, O'Brien JT. Temporal lobe atrophy on MRI in Parkinson disease with dementia: a comparison with Alzheimer disease and dementia with Lewy bodies. Neurology. 2005;64:861–5. doi: 10.1212/01.WNL.0000153070.82309.D4. [DOI] [PubMed] [Google Scholar]
- Thompson PM, Hayashi KM, de Zubicaray G, Janke AL, Rose SE, Semple J, Herman D, Hong MS, Dittmer SS, Doddrell DM, Toga AW. Dynamics of gray matter loss in Alzheimer's disease. J Neurosci. 2003;23:994–1005. doi: 10.1523/JNEUROSCI.23-03-00994.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson PM, Hayashi KM, De Zubicaray GI, Janke AL, Rose SE, Semple J, Hong MS, Herman DH, Gravano D, Doddrell DM, Toga AW. Mapping hippocampal and ventricular change in Alzheimer disease. Neuroimage. 2004;22:1754–66. doi: 10.1016/j.neuroimage.2004.03.040. [DOI] [PubMed] [Google Scholar]
- Warrington EK, James M. The Visual Object and Space Perception Battery. Thames Valley Test Company; Bury St Edmonds: 1991. [Google Scholar]
- Williams LN, Seignourel P, Crucian GP, Okun MS, Rodriguez RL, Skidmore FM, Foster PS, Jacobson C.E.t., Romrell J, Bowers D, Fernandez HH. Laterality, region, and type of motor dysfunction correlate with cognitive impairment in Parkinson's disease. Mov Disord. 2007;22:141–5. doi: 10.1002/mds.21220. [DOI] [PubMed] [Google Scholar]
- Williams-Gray CH, Evans JR, Goris A, Foltynie T, Ban M, Robbins TW, Brayne C, Kolachana BS, Weinberger DR, Sawcer SJ, Barker RA. The distinct cognitive syndromes of Parkinson's disease: 5 year follow-up of the CamPaIGN cohort. Brain. 2009;132:2958–69. doi: 10.1093/brain/awp245. [DOI] [PubMed] [Google Scholar]
- Xu Y, Valentino DJ, Scher AI, Dinov I, White LR, Thompson PM, Launer LJ, Toga AW. Age effects on hippocampal structural changes in old men: The HAAS. Neuroimage. 2007 doi: 10.1016/j.neuroimage.2007.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]