Table 1.
Optimization of photocatalytic trifluoromethylation of 1,1-diphenylethene 2a.a
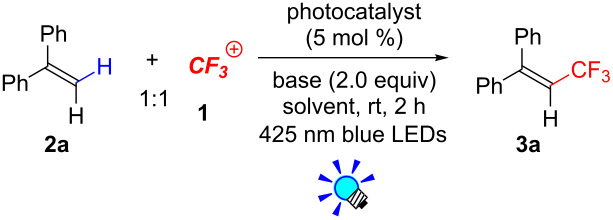 | |||||
| Entry | Photocatalyst | CF3 reagent | Solvent | Base | NMR yield (%) |
| 1 | fac-Ir(ppy)3 | 1a | [D6]-DMSO | K2HPO4 | 82 |
| 2 | fac-Ir(ppy)3 | 1b | [D6]-DMSO | K2HPO4 | 17 |
| 3 | fac-Ir(ppy)3 | 1c | [D6]-DMSO | K2HPO4 | 47 |
| 4 | fac-Ir(ppy)3 | 1a | CD3CN | K2HPO4 | 57 |
| 5 | fac-Ir(ppy)3 | 1a | CD2Cl2 | K2HPO4 | 22 |
| 6 | fac-Ir(ppy)3 | 1a | [D6]-acetone | K2HPO4 | 29 |
| 7 | fac-Ir(ppy)3 | 1a | [D6]-DMSO | none | 81 |
| 8 | [Ru(bpy)3](PF6)2 | 1a | [D6]-DMSO | none | 85 |
| 9 | none | 1a | [D6]-DMSO | none | 0 |
| 10b | [Ru(bpy)3](PF6)2 | 1a | [D6]-DMSO | none | 0 |
aFor reaction conditions, see the Experimental section. bIn the dark.
