Table 3.
Hydrogenation of benzoyl protected enal 9.
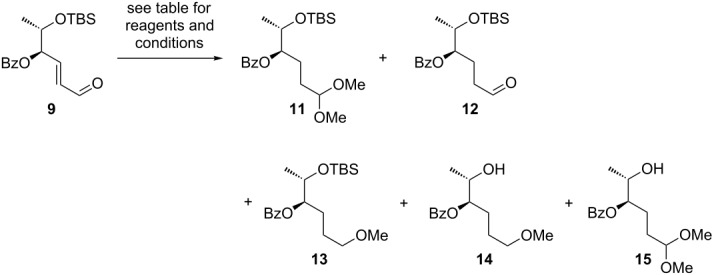 | ||||||
| entry | reagents and conditions | 11 | 12 | 13 | 14 | 15 |
| 1 | Pd/C (10 wt %; 1.6 mol %), H2 (1 bar), methanol, 20 °C, 12 h | 31% | – | 16% | 16% | 8% |
| 2 | Pd(OAc)2 (1 mol %), activated charcoal (9 mol %), H2 (1 bar), methanol, 20 °C, 12 h | 28% | 9% | trace | – | – |
| 3a | Pd(OH)2/C (10 wt %; 1.2 mol %), H2 (1 bar), methanol, 20 °C, 12 h | 51% | 32% | – | – | – |
aProducts 11 and 12 were obtained as an inseparable mixture, yields are estimated from 1H NMR spectrum.
