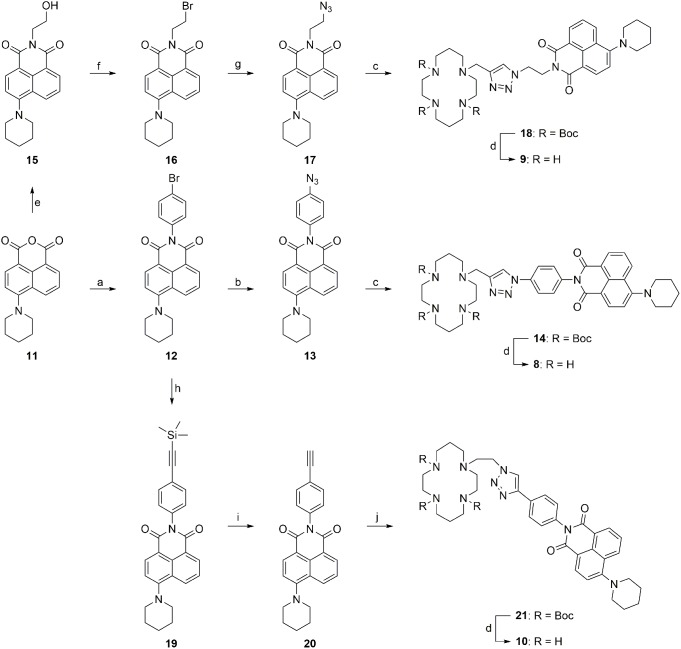
Figure 3. Synthesis of the cyclam-piperidinylnaphthalimide conjugates 8–10.
Reagents and conditions: (a) 4-bromoaniline, piperidine, 2-methoxyethanol, reflux, 72 h, 90%; (b) NaN3, CuI, sodium ascorbate, DMEDA, THF/H2O (7∶3), 12 h, 50%; (c) propargyl-tri-Boc cyclam, CuSO4·5H2O, sodium ascorbate, THF/H2O (7∶3), rt for 13 and 50°C for 17, 12 h, 14: 96%, 18: 92%; (d) (i) TFA/DCM/H2O (90∶5∶5), rt, 6 h; (ii) Ambersep 900 hydroxide form, CH3OH, rt, 15 min, 8: 96%, 9: 99%, 10: 99%; (e) 2-aminoethanol, EtOH, reflux, 22 h, 92%; (f) PBr3, pyridine, THF, 50°C, 16 h, 60%; (g) NaN3, EtOH, reflux, 6 h, 80%; (h) trimethylsilylacetylene, CuI, triphenylphosphine, Pd(PPh3)4, Et3N, pyridine, 85°C, o/n, 94%; (i) K2CO3, CH3OH, rt, o/n, 97%; (j) 2-azidoethyl-tri-Boc cyclam, CuSO4·5H2O, sodium ascorbate, THF/H2O (7∶3), 12 h, 66%.