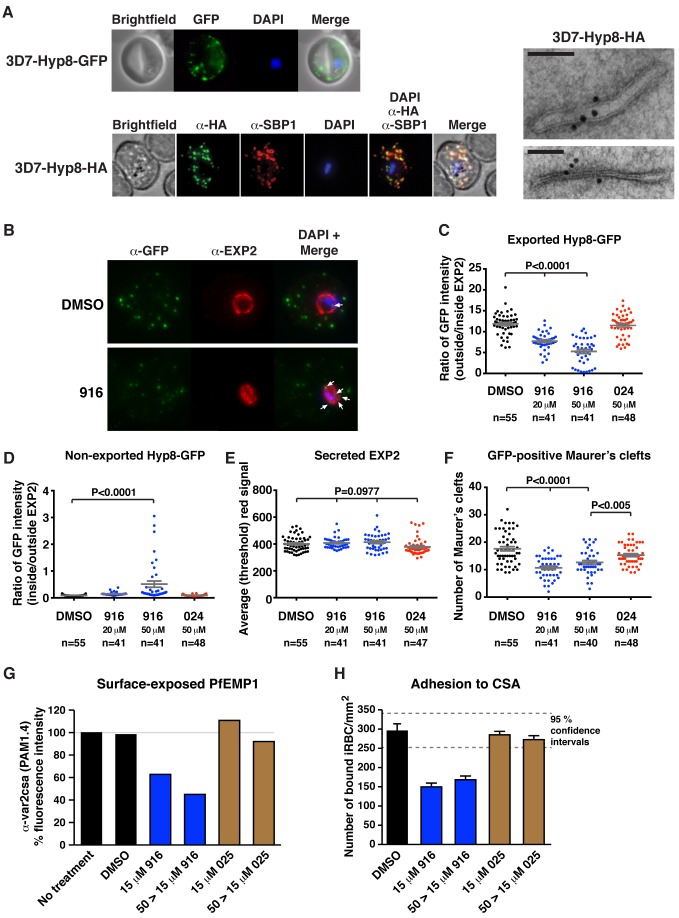
Figure 6. PMV inhibition impairs protein export, PfEMP1 display, and cytoadherence.
(A) (Top) Immunofluorescent micrographs show Hyp8-GFP is exported and localizes within puncta in the infected erythrocyte. (Middle) Hyp8-HA localizes within SBP1-containing MCs. (Right) Immunoelectron microscopy shows Hyp8-HA localization at MCs. Scale bar is 100 nm. (B) Maximum intensity projection micrographs showing export of Hyp8-GFP to MCs and secretion of EXP2 to the parasitophorous vacuole membrane following treatment with DMSO or 916 (50 µM). Puncta of nonexported GFP within the parasite and vacuole is shown (arrows). (C) GFP intensity in MCs (outside EXP2 signal) and within the parasite and parasitophorous vacuole (inside EXP2 signal) was quantified following drug treatment (see Materials and Methods) and is presented as a ratio: exported = ratio outside/inside EXP2. The number of infected cells counted (n) is shown. (D) GFP within the parasite and parasitophorous vacuole (inside EXP2 signal) was quantified across treatments and is presented as a ratio: nonexported = ratio inside/outside EXP2. (E) EXP2 intensity in the parasitophorous vacuole membrane (red) was quantified across treatments and is presented as average (threshold) signal. (F) The number of GFP-positive MCs per infected erythrocyte was quantified across treatments. Error bars represent the mean ±SEM, and p values were determined by ANOVA in (C–F). (G) Surface-exposed PfEMP1 (VAR2CSA) on infected erythrocytes was measured following inhibitor treatment by FACS using monoclonal human PAM1.4 serum [30]. Geometric mean fluorescence of >100,000 cells per condition are shown relative to no treatment. Parasites received inhibitor at 15 µM for 23 h (15 µM) or 50 µM for 12 h followed by a reduction to 15 µM for 11 h (50>15 µM). Shown is a single representative of duplicate experiments. Raw FACS data are presented in Figure S7. (H) Adhesion of infected red blood cells (iRBCs) to chondroitin sulfate A (CSA) under static conditions. Adherent iRBCs were counted in ten 0.28 mm2 fields of view per sample from triplicate samples and are shown as number of iRBCs per mm2. Shown is a single representative of duplicate experiments. Data represent mean ±SEM. 95% confidence intervals are shown (grey dashed lines). 916 significantly reduced adhesion to CSA p<.0001.