Abstract
Determining the generic delimitations within Aeridinae has been a significant issue in the taxonomy of Orchidaceae, and Holcoglossum is a typical case. We investigated the phylogenetic utility of the morphological traits of leaf epidermis in the taxonomy of Holcoglossum s.l. by using light and scanning electron microscopy to analyze 38 samples representing 12 species of Holcoglossum, with five species from five closely related genera, such as Ascocentrum, Luisia, Papilionanthe, Rhynchostylis and Vanda. Our results indicated that Holcoglossum can be distinguished from the related genera based on cuticular wax characteristics, and the inclusion of Holcoglossum himalaicum in Holcoglossum is supported by the epidermis characteristics found by LM and SEM. The percentage of the tetracytic, brachyparacytic, and laterocytic stomata types as well as the stomata index and certain combinations of special wax types support infrageneric clades and phylogenetic relationships that have been inferred from molecular data. Laterocytic and polarcytic stomata are perhaps ecological adaptations to the strong winds and ample rains in the alpine region of the Hengduanshan Mountains.
Introduction
Determining the generic delimitations within orchid subtribe Aeridinae is difficult and has been considered a “black pit” [1]–[7] and the genus Holcoglossum Schltr. is such an example [3], [5], [8]. The orchid genus Holcoglossum (Aeridinae, Vandeae, Orchidaceae) is comprised of approximately 17 species ranging from Malaysia, Thailand, Myanmar, Vietnam and northeastern Yunnan, and eight of which are endemic to China [9], and it is a key genus in the “Vanda-Aerides” alliance, which includes Aerides, Ascocentrum, Ascolabium, Holcoglossum, Neofinetia, Papilionanthe, Penkimia, Seidenfadenia and Vanda [10]. Since its establishment in 1919 [11], the genus has had several circumscriptions [3]–[5], [10]–[21]. Recent molecular phylogenetic studies have shed new light on the generic delimitation of Holcoglossum and its alliances. However, several different taxonomic treatments have been proposed based on molecular phylogenetic research [13], [20], [21]. A recent molecular phylogenetic study suggested that there are 17 species in Holcoglossum s.l., including 14 species of Holcoglossum s.s., and three species from its alliance, including Ascocentrum himalaicum (Deb. Sengupta & Malick) Christenson, Ascolabium pumilum (Hayata) Schltr., and Penkimia nagalandensis Phukan & Odyuo [13].
In addition to debates on the generic delimitation of Holcoglossum, several perspectives exist on the infrageneric taxonomy of this genus [10], [12], [13], [20], [21]. Christenson [10] divided Holcoglossum into two sections based on the flower number per inflorescence and plant form. Jin [12] proposed an infrageneric system based on floral characteristics, such as the callus, spur and stipe; this system has been supported by the results of recent molecular phylogenetic studies [13], [20]. However, four species in Jin's system, H. omeiense X. H. Jin & S. C. Chen, H. lingulatum (Averyanov) Averyanov, H. wangii Christenson and H. kimballianum (Rchb. f.) Garay in H. sect. Holcoglossum, share similar habits and floral characteristics, including a crested callus and long spur, are grouped into more distant clades in phylograms in molecular phylogenetic studies. The search for morphological synapomorphies for each monophyletic clade has been problematic because of widespread homoplasy [13], [20]. And anatomical studies of leafless Vandeae have revealed an extreme homoplasy in leaf and root characteristics with no clear evolutionary trend, even among genera [23].
In an attempt to overcome these difficulties, previously underexplored morphological characteristics are now being studied in more depth, such as characteristics of pollinia and leaf epidermis. Although pollinia characteristics (number and aperture) are largely homoplasious for Aeridinae in total, they are often useful at the generic and/or infrageneric level. For instance, Neofinetia and Holcoglossum have porate pollinia, whereas Vanda, Rhyncostylis, Aerides and Trudelia have cleft pollinia [7], [13], [22]. The mature leaf epidermal characteristics, such as the pattern of epidermal cells, type of stomata, shape of guard cell pairs and ornamentation of cuticles, are considered useful in the phylogenetic studies at the level of genera or subgenera in angiosperms [24], [25]. However, the use of such characteristics varies in importance in the phylogenetic study of Orchidaceae, and it has played a minor role in studies of leaf anatomy in Orchidaceae in recent years [26]–[31]. Some authors suggest that the development of the stomata is required to understand the phylogenetic importance of stomatal characteristics because different development pathways may result in the same stomatal type [27]. However, the stomata type as a systematic characteristic has been used to divide the Orchidaceae taxonomy and Orchidaceae into four divisions: Anomocyticeae, Acyticeae, Cyclocyticeae and Paracyticeae [32]. Tomlinson [27] suggested that the stomata complex has value as a systematic characteristic only in combination with a variety of other characterristics and only in groups that are already known to be natural.
Recent studies have indicated that the ontogeny of stomata in Holcoglossum and its relatives is the perigenous, which is similar to most of Orchidaceae, and tetracytic type of stomata (four somewhat equally sized subsidiary cells around the stoma) is the most popular stomatotype [27], [29], [30]. Furthermore, Holcoglossum is a natural group suggested by molecular phylogenetic studies [13], [20]. Hence, it is reasonable to consider the phylogenetic significance of the mature leaf characteristics without studying the development of stomata in Holcoglossum. The aim of the present study is to investigate whether the characteristics of mature leaf epidermis can provide additional taxonomic and phylogenetic information for the genus Holcoglossum and the infrageneric clades that have been suggested by recent results of molecular phylogenetics.
Results
The leaf epidermal characteristics are listed in Tables 1, 2, 3. The molecular phylogeny of the Holcoglossum infrageneric clades mentioned below includes three clades from Xiang et al. [13]: TC (tropic clade, including H. amesianum (Rchb. f.) Christenson, H. subulifolium (Rchb. f.) Christenson, H. wangii, H. kimballianum, H. nagalandense (Phukan & Odyuo) X.H. Jin, and H. himalaicum (Deb, Sengupta & Malick) Aver.), AC (the temperate alpine clade, including H. flavescens (Schltr.) Z.H. Tsi, H. rupestre (Hand.-Mazz.) Garay, H. sinicum Christenson, H. weixiense X.H. Jin & S.C. Chen, H. tsii T. Yukawa and H. nujiangense X.H. Jin & S.C. Chen), and HC (clade located between TC and AC, including H. omeiense, H. lingulatum, H. quasipinifolium (Hayata) Schltr., and H. pumilum (Hayata) X.H. Jin).
Table 1. Leaf epidermal characteristics of Holcoglossum, its relatives and outgroup under LM.
| Taxa | Shape of cells | Pattern of anticlinal walls | Stomatal apparatus | Size of stoma (L/W) | Stoma index (%) | Stoma grouping (%) | Figures |
| (Ad/Ab) | |||||||
| Holcoglossum nujiangense | Pol(4)/Pol(4) | Str/Str | Exist/Exist | 1.2/0.9 | 2.6/2.8 | 0/28.6 | Fig 3, B–C |
| H. weixiense | Pol(4)/Pol(4) | Str/Str | Exist/Exist | 1.4/1.2 | 2.1/2.8 | 30.8/22.9 | Fig 3, D–E |
| H. sinicum | Pol(4)/Pol(4) | Str/Str | Exist/Exist | 1.1/1.0 | 2.2/3.1 | 7.7/11.3 | Fig 2, G–H |
| H. rupestre | Pol(4)/Pol(4) | Str/Str | Exist/Exist | 1.1/1.0 | 2.0/2.6 | 31.3/23.6 | Fig 2, I; Fig 3 A |
| H. flavescens | Pol(4–6)/Pol(4–6) | Str/Str | Exist/Exist | 0.9/1.0 | 2.0/2.6 | 7.1/21.4 | Fig 2, E–F |
| H. omeiense | Pol(4–6)/Pol(4–6) | Str/Str | Exist/Exist | -/0.9 | -/4.3 | -/19.8 | Fig 3, H–I |
| H. lingulatum | Pol(4–6)/Pol(4–6) | Str/Str | Exist/Exist | -/1.1 | -/4.0 | -/16.5 | Fig 3, F–G |
| H. himalaicum | Pol(6)/Pol(6) | Str/Str | Exist/Exist | 1.3/1.1 | 2.0/2.1 | 0/0.6 | Fig 4, C–D |
| H. wangii | Pol(6)/Pol(6) | Str/Str | Exist/Exist | 0.9/1.0 | 0.6/0.7 | 0/0 | Fig 1, G–H |
| H. kimballianum | Pol(6)/Pol(6) | Str/Str | Exist/Exist | 1.2/1.3 | 0.6/0.7 | 0/0 | Fig 1, E–F |
| H. amesianum | Pol(6)/Pol(6) | Str/Str | Exist/Exist | 1.0/1.0 | 1.9/2.2 | 0.5/0.5 | Fig 1, A–B |
| H. subulifolium | Pol(6)/Pol(6) | Str/Str | Exist/Exist | 1.1/1.1 | 2.1/1.4 | 0/0 | Fig 1, C–D |
| Papilionanthe biswasiana | Pol(4–6)/Pol(4–6) | Str-arc/Str-arc | Exist/Exist | 1.3/1.3 | 7.5/7.5 | 25.0/25.0 | Fig 1, I |
| Ascocentrum ampullaceum | Pol(4–6)/Pol(6) | Str-arc/Str-arc | Exist/Exist | -/1.4 | -/2.5 | -/3.1 | Fig 2, A–B |
| Vanda pumila | Pol(4–6)/Pol(4) | Str/Str | Absent/Exist | 0/1.3 | 0/2.6 | 0/0 | Fig 2, C–D |
| Rhynchostylis retusa | Pol(6)/Pol(6) | Str/Str | Exist/Exist | 1.3/1.4 | 3.8/4 | 0/1.5 | Fig 4, A–B |
| Luisia magniflora | Pol(4–6)/Pol(4–6) | Str-arc/Str-arc | Exist/Exist | 1.2/1.2 | 3.1/3.1 | 5.8/5.8 | Fig 4, E |
Pol(4), polygon (quadrilateral); Pol(6), polygon (hexagonal); Pol(4–6), polygon (quadrilateral to hexagonal); Str, straight; Str-arc, straight to arched; Ad/Ab, adaxial epidermis/abaxial epidermis; -, no data analysis.
Table 2. Stomatotypes and substomatotypes of Holcoglossum, its relatives and outgroup under LM.
| Taxa | Stomatotype (%) Ad/Ab | ||||||||||||||
| Tetra | Para | Latero | Polar | ||||||||||||
| Hemipara | Brachypara | 1+2 | 2+2 | 3+(1–4) | Total | 1+2 | 2+2 | 3+(1–4) | Total | 5(1+2) | 6(2+2) | ≥6(3+{?1–4}?) | Total | ||
| Holcoglossum nujiangense | 9.1/0 | 0/0 | 0/0 | 9.1/0 | 0/0 | 0/0 | 9.1/0 | 18.2/42.9 | 27.3/28.6 | 36.4/0 | 81.8/71.4 | 0/14.3 | 0/0 | 0/14.3 | 0/28.6 |
| H. weixiense | 8.7/0 | 0/0 | 4.3/0 | 17.4/16.1 | 17.3/3.2 | 0/0 | 39.1/19.4 | 13.0/25.8 | 30.4/12.9 | 8.7/16.1 | 52.1/54.8 | 0/6.5 | 0/6.5 | 0/12.9 | 0/25.8 |
| H. sinicum | 9.1/9.1 | 0/0 | 18.2/3.0 | 18.2/3.0 | 4.5/0 | 0/3.0 | 40.9/9.1 | 18.2/12.2 | 0/18.2 | 9.1/15.2 | 27.3/45.6 | 0/9.1 | 9.1/6.1 | 13.6/21.2 | 22.7/36.4 |
| H. rupestre | 4.2/4.2 | 0/0 | 20.8/3.8 | 12.5/7.7 | 4.2/0 | 0/0 | 37.5/11.5 | 8.0/25.0 | 8.3/5.8 | 20.8/26.9 | 37.1/57.7 | 4.2/7.7 | 4.2/0 | 12.5/19.2 | 20.8/26.9 |
| H. flavescens | 14.3/5.0 | 0/0 | 8.6/3.0 | 14.3/7.0 | 2.9/4.0 | 5.7/3.0 | 31.5/17.0 | 8.6/14.0 | 5.7/9.0 | 0/7.0 | 14.3/30.0 | 22.9/28.0 | 5.7/10.0 | 11.4/10.0 | 40.0/48.0 |
| H. omeiense | -/8.4 | 0/0 | -/3.4 | -/9.2 | -/3.4 | -/0 | -/15.7 | -/13.8 | -/9.2 | -/9.2 | -/32.2 | -/19.5 | -/10.3 | -/13.8 | -/43.7 |
| H. lingulatum | -/11.5 | 0/0 | -/9.8 | -/1.6 | -/1.7 | -/0 | -/13.1 | -/18.1 | -/5.0 | -/4.9 | -/27.9 | -/29.5 | -/4.9 | -/13.1 | -/47.5 |
| H. himalaicum | 50.7/68.6 | 0/0 | 30.7/21.4 | 5.3/0 | 0/0 | 0/0 | 36.0/21.4 | 6.7/5.7 | 2.7/0 | 2.7/1.4 | 12.1/7.1 | 1.3/0 | 0/2.9 | 0/0 | 1.3/2.9 |
| H. wangii | 46.2/37.1 | 0/0 | 15.3/20.0 | 0/5.7 | 0/0 | 0/0 | 15.4/25.7 | 15.3/20.0 | 7.7/8.6 | 7.7/0 | 30.8/28.6 | 7.7/8.6 | 0/0 | 0/0 | 7.7/8.6 |
| H. kimballianum | 58.3/31.2 | 0/0 | 37.5/30.3 | 0/0.9 | 0/1.8 | 0/0 | 37.5/33.0 | 0/8.3 | 0/4.6 | 0/0 | 0/12.9 | 4.2/14.7 | 0/4.6 | 0/3.7 | 4.2/22.9 |
| H. amesianum | 43.1/31.1 | 0/0 | 45.1/33.3 | 0/0 | 0/0 | 0/0 | 45.1/33.3 | 7.8/22.2 | 2.0/2.2 | 0/2.2 | 9.8/26.7 | 2.0/6.7 | 0/2.2 | 0/0 | 2.0/8.9 |
| H. subulifolium | 63.8/56.5 | 0/0 | 23.4/27.2 | 0/0 | 0/0 | 0/0 | 23.4/27.2 | 0/14.1 | 0/1.1 | 0/0 | 0/15.2 | 12.8/1.1 | 0/0 | 0/0 | 12.8/1.1 |
| Papilionanthe biswasiana | 0/0 | 0/0 | 38.4/38.4 | 23.1/23.1 | 23.1/23.1 | 0/0 | 38.4/38.4 | 15.4/15.4 | 0/0 | 0/0 | 61.5/61.5 | 0/0 | 0/0 | 0/0 | 0/0 |
| Ascocentrum ampullaceum | -/14.9 | 0/0 | -/7.5 | -/10.4 | -/4.5 | -/0 | -/22.4 | -/31.3 | -/13.4 | -/1.5 | -/46.2 | -/9.0 | -/6.0 | -/1.5 | -/16.5 |
| Vanda pumila | 0/4.5 | 0/0 | 0/22.7 | 0/0 | 0/4.5 | 0/0 | 0/27.2 | 0/18.2 | 0/22.7 | 0/4.5 | 0/45.4 | 0/0 | 0/13.6 | 0/9.1 | 0/22.7 |
| Rhynchostylis retusa | 30.0/26.5 | 0/0 | 60.0/63.8 | 0/0 | 0/0 | 0/0 | 60.0/63.8 | 10.0/9.6 | 0/0 | 0/0 | 10.0/9.6 | 0/0 | 0/0 | 0/0 | 0/0 |
| Luisia magniflora | 0/0 | 6.3/6.3 | 84.8/84.8 | 0.9/0.9 | 0.4/0.4 | 0/0 | 92.4/92.4 | 4.9/4.9 | 1.3/1.3 | 0/0 | 6.2/6.2 | 1.3/1.3 | 0/0 | 0/0 | 1.3/1.3 |
Ad/Ab, adaxial epidermis/abaxial epidermis; tetra, para, latero and polar, represent tetracytic, paracytic, laterocytic and polarcytic respectively; para 1+2 indicates the distribution of the subsidiary cells (in this case, 3 subsidiary cells with1 on one lateral side of the stoma and 2 on the other side); latero 1+2 (same meaning as para 1+2); polar 5(1+2) indicates the distribution of the subsidiary cells (in this case, 5 subsidiary cells with 2 polar not inlcluded in the name of type and 3 lateral,1+2 represents 3 lateral with same meaning as para 1+2); -, no data analysis. Each species is represented in Figure 1.
Table 3. Wax types of the leaf epidermides of Holcoglossum, its relatives and outgroup under SEM.
| Taxa | Wax Types(Ad) | Wax Types(Ab) | Figures | |||||||||||||||
| 1 | 2 | 3 | 3* | 3** | 4 | 4* | 5 | 6 | 1 | 2 | 3 | 3* | 3** | 4 | 4* | 5 | ||
| Holcoglossum sinicum | - | ▴ | - | - | ▴ | - | - | - | ▴ | - | - | ▴ | - | - | - | Fig 7, E–F | ||
| H. nujiangense | - | ▴ | - | ▴ | - | - | - | ▴ | - | ▴ | - | ▴ | - | ▴ | - | ▴ | Fig 7, C–D | |
| H. flavescens | - | ▴ | - | ▴ | - | ▴ | - | ▴ | - | ▴ | - | - | ▴ | ▴ | - | ▴ | Fig 7, A–B | |
| H. omeiense | - | ▴ | - | - | ▴ | ▴ | - | - | - | ▴ | - | - | ▴ | ▴ | - | - | Fig 6, E–F | |
| H. lingulatum | - | ▴ | - | - | ▴ | ▴ | - | - | - | ▴ | - | - | ▴ | ▴ | - | - | Fig 6, C–D | |
| H. himalaicum | ▴ | ▴ | ▴ | ▴ | ▴ | Fig 9, C–D | ||||||||||||
| H. kimballianum | - | ▴ | - | ▴ | - | ▴ | - | - | - | ▴ | - | ▴ | - | ▴ | - | - | Fig 6, A–B | |
| H. wangii | - | ▴ | ▴ | - | - | ▴ | - | - | - | ▴ | ▴ | - | - | ▴ | - | - | Fig 5, E–F | |
| H. subulifolium | - | ▴ | ▴ | - | - | ▴ | - | - | - | ▴ | - | ▴ | - | ▴ | - | - | Fig 5, C–D | |
| H. amesianum | - | ▴ | - | ▴ | - | ▴ | - | - | - | ▴ | - | ▴ | - | ▴ | - | - | Fig 5, A–B | |
| Papilionanthe biswasiana | - | ▴ | - | - | ▴ | - | ▴ | - | - | ▴ | - | - | ▴ | - | ▴ | - | Fig 8, E | |
| Ascocentrum ampullaceum | ▴ | ▴ | - | - | - | - | - | - | ▴ | ▴ | - | - | - | - | - | - | Fig 8, A–B | |
| Vanda pumila | ▴ | ▴ | - | ▴ | - | - | - | - | ▴ | ▴ | - | - | - | - | - | - | Fig 8, C–D | |
| Rhynchostylis retusa | ▴ | ▴ | -/▴ | ▴ | ▴ | Fig 9, A–B | ||||||||||||
| Luisia maniflora | ▴ | ▴ | ▴ | ▴ | Fig 9, E | |||||||||||||
Ad/Ab, adaxial epidermis/abaxial epidermis; 1, crusts; 2, granules; 3, sparse platelets; 3*, medium platelets; 3**, dense platelets; 4, flat rodlets; 4*, distinct rodlets; 5, threads; 6, membrancous platelets; ▴, with this character; -, without this character.
Epidermal cells
When viewed under LM, the epidermal cells on a single leaf including cells connected to stomata and those around it, have the same shape although narrower in some depressed strip areas that are parallel to the length of the leaf, and it is usually polygonal in form in all of the sample materials in this study. In Holcoglossum, the shape is hexagonal in the TC clade, quadrilateral to hexagonal in the HC clade and H. flavescens of the AC clade, and quadrilateral in all of the other species of the AC clade (Figs. 1, 2, 3, 4). When examined under SEM, the anticlinal walls of the epidermal cells are obscure or invisible under thick waxes (Figs. 5, 6, 7, 8, 9).
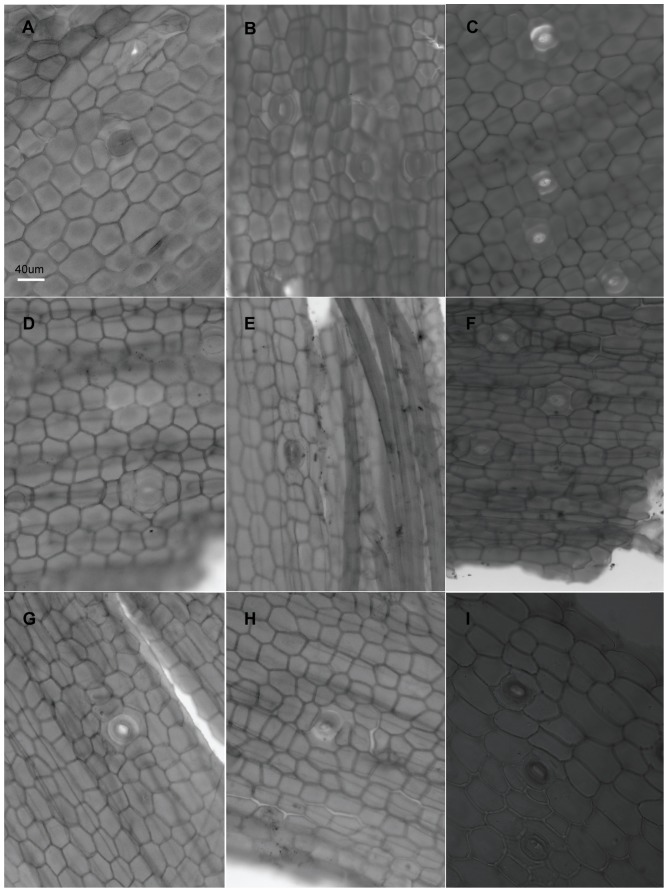
Figure 1. Characteristics of the epidermal cells (LM).
A–B. Holcoglossum amesianum. A, adaxial, tetracytic; B, abaxial, brachyparacytic and laterocytic 1+2. C–D. H. subulifolium. C, adaxial, tetracytic and brachyparacytic; D, abaxial, tetracytic. E–F. H. kimballianum. E, adaxial, tetracytic; F, abaxial, tetracytic. G–H. H. wangii. G, adaxial, tetracytic; H, abaxial, tetracytic. I. Papilionanthe biswasiana, brachyparacytic and laterocytic 1+2. B–I have the same scale bars as in A.
Figure 2. Characteristics of the epidermal cells (LM).
A–B. Ascocentrum ampullaceum. A, adaxial; B, abaxial, polarcytic 6 (2+2) and polarcytic 5 (1+2). C–D. Vanda pumila. C, adaxial; D, abaxial, brachyparacytic and polarcytic 6 (2+2). E–F. Holcoglossum flavescens. E, adaxial, tetracytic, polarcytic 5(1+2) and polarcytic 6 (2+2); F,abaxial, polarcytic 5 (1+2) and laterocytic 3+4. G–H. H. sinicum. G, adaxial, paracytic and laterocytic 1+2; H, abaxial, polarcytic 5 (1+2) and polarcytic 5 (1+2). I. H. rupestre, adaxial, polarcytic 5 (1+2). Scales are the same as in Fig 2.
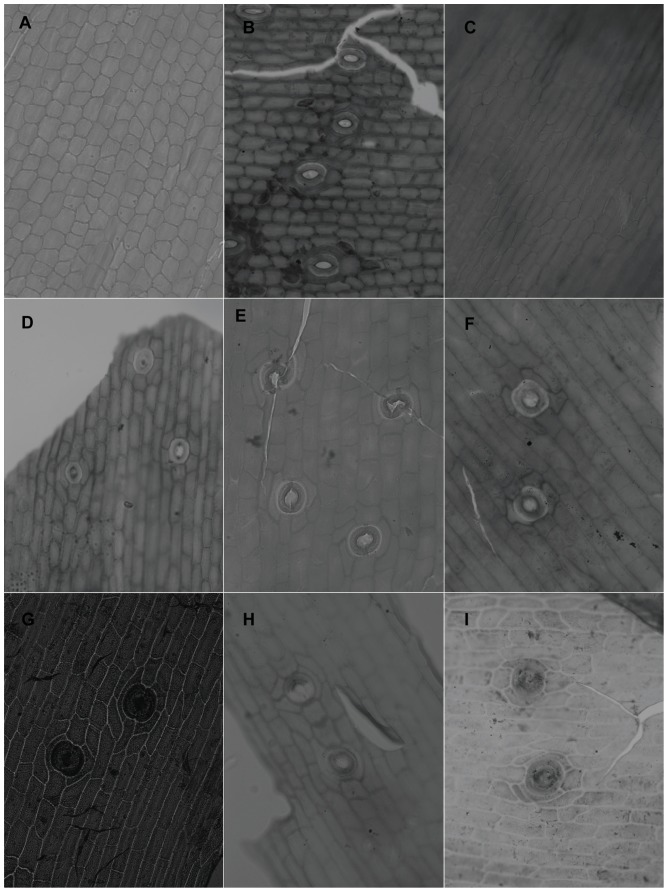
Figure 3. Characteristics of the epidermal cells (LM).
A. Holcoglossum rupestre, abaxial, tetracytic and polarcytic 6(1+3). B–C. H. nujiangense. B, adaxial, laterocytic 1+2; C, abaxial, polarcytic 6 (2+2). D–E. H. weixiense. D, adaxial, tetracytic and laterocytic 2+3; E, abaxial, paracytic 2+2, laterocytic 1+3, polarcytic 6(1+3) and polarcytic 8(3+3). F–G. H. lingulatum. F, adaxial, paracytic 2+2; G, abaxial, tetracytic, brachyparacytic, polarcytic 5 (1+2) and polarcytic 6(2+2). H–I. H. omeiense. H, adaxial; I, abaxial, tetracytic. Scales are the same as in Fig 2.
Figure 4. Characteristics of the epidermal cells (LM).
A–B. Holcoglossum himalaicum. A, adaxial, tetracytic; B, abaxial, tetracytic. C–D. Rhynchostylis retusa. C, adaxial, tetracytic; D, abaxial, tetracytic. E. Luisia magniflora. brachyparacytic. B–E have the same scale bars as in A.
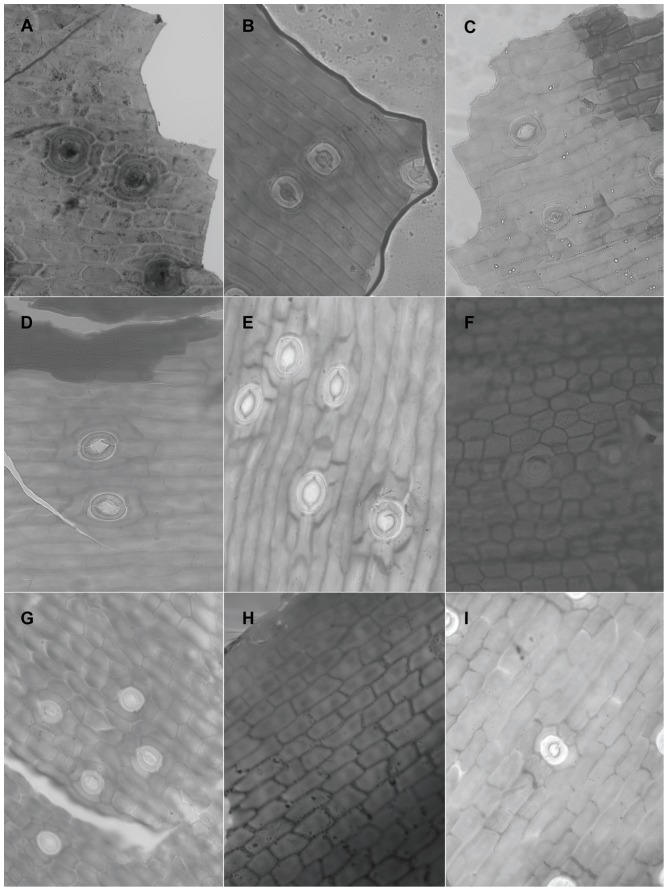
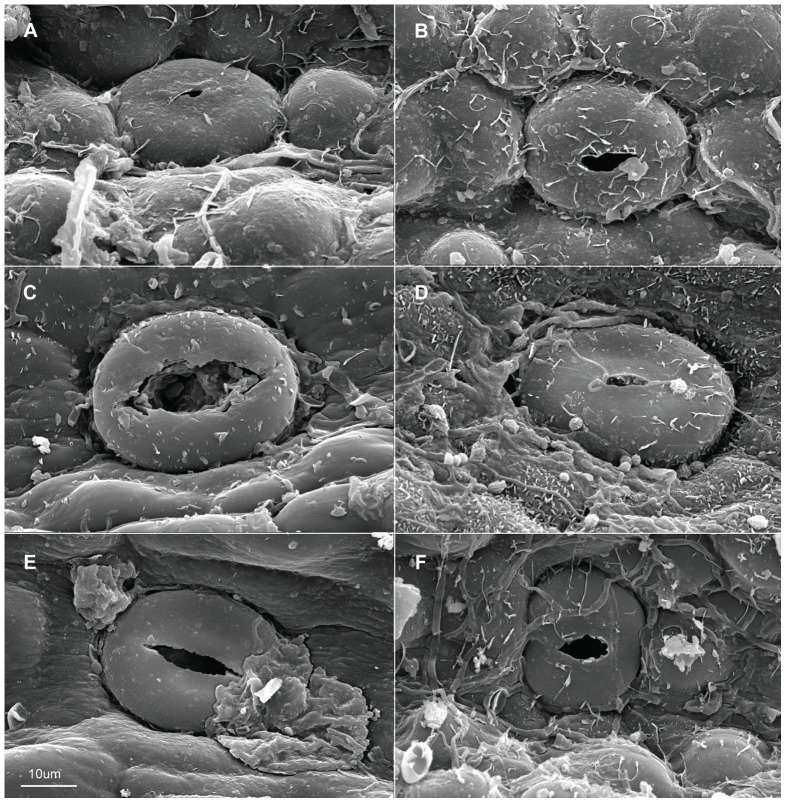
Figure 5. Characteristics of the epidermal cells (SEM).
Adaxial on the left, abaxial on the right. A–B. Holcoglossum amesianum; C–D. H. subulifolium; E–F. H. wangii. A–D, F have the same scale bars as in E.
Figure 6. Characteristics of the epidermal cells (SEM).
Adaxial on the left, abaxial on the right. A–B. Holcoglossum kimballianum; C–D. H. lingulatum; E–F. H. omeiense. Scales are the same as in Fig 6.
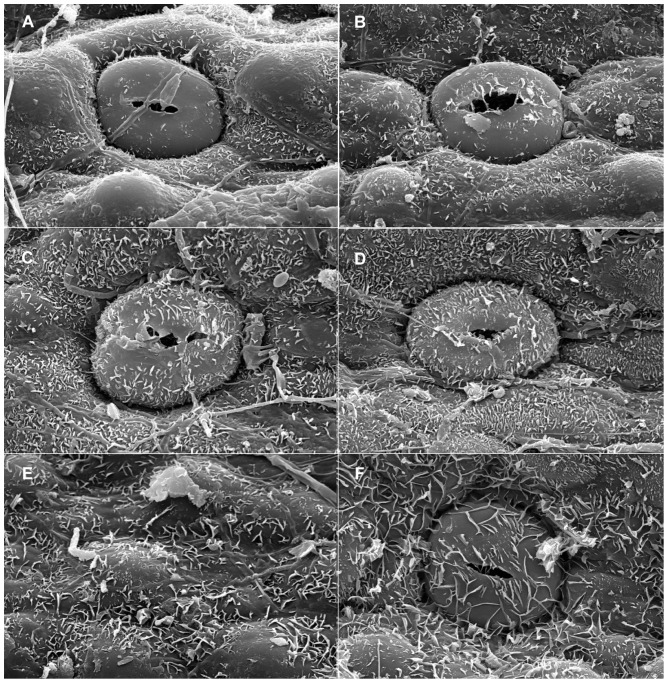
Figure 7. Characteristics of the epidermal cells (SEM).
Adaxial on the left, abaxial on the right. A–B. Holcoglossum flavescens; C–D. H. nujiangense; E–F. H. sinicum. Scales are the same as in Fig 6.
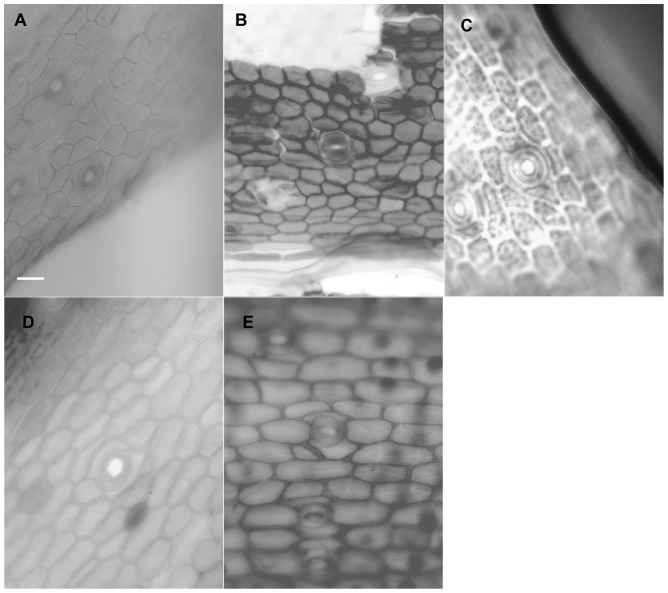
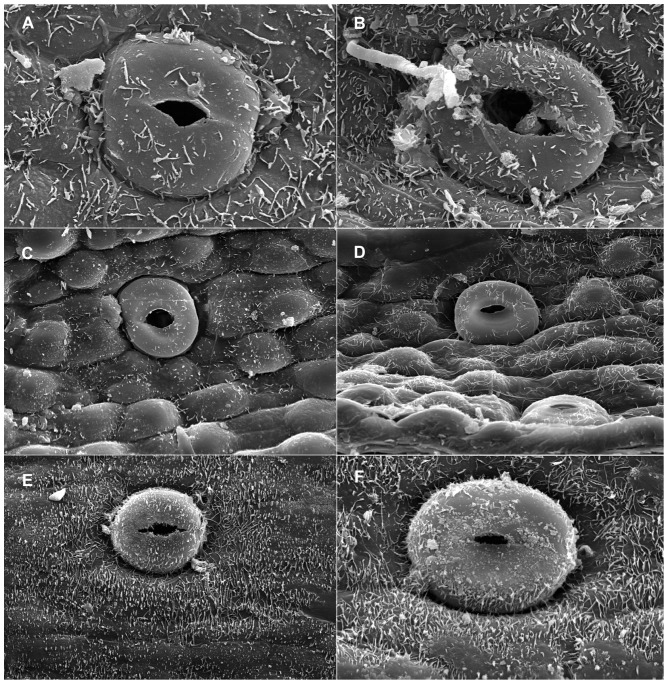
Figure 8. Characteristics of the epidermal cells (SEM).
Adaxial on the left, abaxial on the right. A–B. Ascocentrum ampullaceum; C–D. Vanda pumila; E. Papilionanthe biswasiana. A–D have the same scale bars as in E.
Figure 9. Characteristics of the epidermal cells (SEM).
Adaxial on the left, abaxial on the right. A–B. Rhynchostylis retusa; C–D. Holcoglossum himalaicum; E. Luisia magniflora. A–D have the same scale bars as in E.
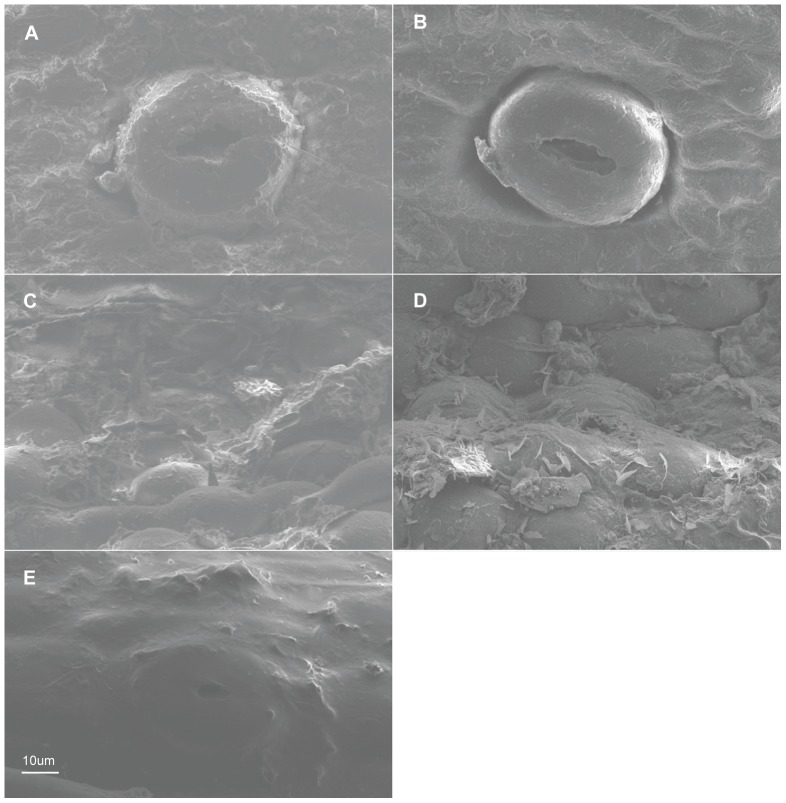
There are six wax types and six varieties: 1, crusts; 2, granules; 3, sparse platelets; 3*, medium platelets; 3** dense platelets; 4, flat rodlets; 4* distinct rodlets; 5, threads; and 6, membranous platelets. Normally, the wax characteristics of the abaxial (Ab) surface are more distinct than those of the adaxial (Ad) surface. Flat rodlets on the abaxial surface of the leaves and threads are only found in Holcoglossum; whereas granules are present in all of the samples (Table 3). In the genus Holcoglossum, flat rodlets are mainly found in the TC and HC clades on both surfaces of the leaves except for H. himalaicum on the abaxial surface (Figs. 5, 6; Fig. 7. A, B, D; Fig 9. D); threads are present in two species of the AC clade on both surfaces of the leaves (Fig. 7. A–D); dense platelets are predominant in the HC clade and some species of the AC clade on both surfaces of the leaves except for H. flavescens on the abaxial surface (Fig. 6. C–F and Fig. 7. B, E, F); and sparse or medium platelets present mainly in the TC clade on both surfaces of the leaves except for H. himalaicum on the abaxial surface (Fig. 5. A–F; Fig. 6. A, B; Fig. 9. D). Trichomes are found in H. himalaicum and Luisia magniflora Z. H. Tsi & S. C. Chen (Fig. 9. C) and secretory cells are occasionally seen in a few species, such as in the adaxial leaf surface of H. subulifolium.
Stomatal apparatus
Stomata are present on both surfaces in all of the samples of Holcoglossum, Rhynchostylis retusa (L.) Bl. and Ascocentrum ampullaceum (Roxb.) Schltr., although rare stomata are present on the Ad surface of H. omeiense, H. lingulatum and A. ampullaceum (Figs. 5, 6, 7; Fig. 8. A, B; Fig. 9. A, B). They are only present on the Ab surface in Vanda pumila Hook. f. (Fig. 8. D). In Papilionanthe biswasiana (Ghose et Mukerjee) Garay and Luisia magniflora, stomata are presented on the entire leaf surface because of its terete leaf (Fig. 8. E and Fig. 9. E).
Stomatal clustering (mostly two stomata) is mainly found in the AC and HC clades and scarcely found in the TC clade of genus Holcoglossum (Fig. 3. A, B, E). Stomatal clustering consists of “non-contiguous clusters” formed by groups of stomata that do not have contact with each other (they are separated by the subsidiary cells) [33].
The guard cells are on the same level with the epidermis in the TC clade, are above the epidermis in the AC clade and at a medium level in the HC clade (Figs. 5, 6, 7 and Fig. 9. C, D).
Stomata types
The stomata on a single leaf conform to several types, including tetracytic, paracytic, laterocytic or polarcytic. Other very rare stomatotypes found in several species are not included in the total of the stomatotypes; these stomatotypes include the hexacytic on the Ab surface of A. ampullaceum, H. lingulatum, and H. flavescens and Ad surface of H. weixiense and the stephanocytic type on both surfaces of H. flavescens. H. rupestre, and P. biswasiana and Ab surface of H. lingulatum. The determination of the extent of the variability, the predominant stomatotype and the proportion of these types is required so that the stomatal characteristics can be properly correlated with other characteristics, which would be useful for classification purposes [34].
In Holcoglossum, the tetracytic type is present in all of the samples except on the Ab surface of H. nujiangense and H. weixiense. This type has value scopes on the Ab surface of 31.1–68.6% in TC, 0–9.1% in AC and 8.4–11.5% in HC. The brachyparacytic type on the Ab surface has similar value scopes, with 20.0–38.4% in TC, 0–3.8% in AC and 3.4–9.8% in HC. The laterocytic type on the Ab surface has a reverse tendency with 12.9–28.6% in TC, 27.9–32.2% in HC, and 30.0–71.4% in AC (Table 2). Because there is infrequent stomata on the Ad surface of H. lingulatum and H. omeiense, the percentage of the stomatal types in these two species has little significance. Except for these two species, the percentage of tetracytic type on the Ad surface has value scopes of 43.1–63.8% in TC and 4.2–12.2% in AC (Table 2).
Stomatal index
The scope of the stomatal index is 0–7.5%/0.7%–7.5% (Ad/Ab). The stomatal index on the Ab surface is divided into two groups, and H. wangii, H. kimballianum, H. himalaicum, H. omeiense and H. lingulatum are divided into two clades with the former three species in the TC clade (0.7%–2.2%) and the latter two in the HC clade (4.0%–4.3%).
Discussion
Systematic significance of the leaf epidermis in the generic delimitation and the subdivision of the genus Holcoglossum
In this study, we found that the wax type is a useful taxonomic characteristic that can distinguish Holcoglossum from its relatives and define infrageneric clades that are recognized in molecular studies [13], [20]. Flat rodlets, threads or only dense platelets in addition to granules on the Ab surface are characteristics of Holcoglossum. Our results lend support to the broad taxonomic treatment of Holcoglossum with infrageneric systems. Our results indicate that stomatal and other epidermal features in the Ab surface of the leaves are constant within species and represent good characteristics for taxonomic and phylogenetic studies.
Infrageneric evolutionary directions and ecological adaptions to the environment
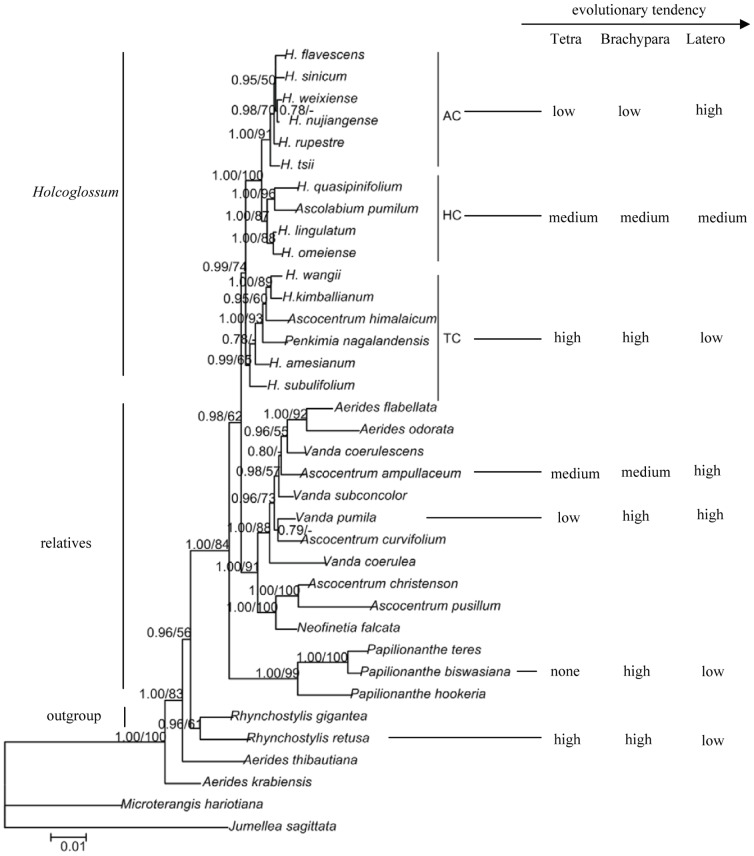
The percent of the stomatotypes on the Ab surface of Holcoglossum has an obvious infrageneric evolutionary tendency, with the percentage of the tetracytic and brachyparacytic types decreasing in the direction of TC>HC>AC, although there are small intersections between HC and AC clades, whereas the percentage of the laterocytic type is in the reverse order with the highest value in the AC clade (Fig. 10). Using Rhynchostylis retusa as outgroup, our results indicated that a high percentage of tetracytic and brachyparacytic types and low percentage of laterocytic types are more basal (Fig. 10). The tetracytic type is a relatively simple type with one subsidiary cell on each lateral side of the stoma, whereas the laterocytic type is a comparatively complex type with more than 2 subsidiary cells on one lateral side or both lateral sides of the stoma. Our results are consistent with Williams' opinion [29] that more than two subsidiary cells are more evolutionarily derived than only two subsidiary cells, with more than four subsidiary cells only found in the most advanced orchids, such as species of Trichocentrum and Erycina. Species of Trichocentrum have fleshy or terete leaves, similar to the channeled semi-terete leaves of Holcoglossum [29].
Figure 10. Molecular phylogeny presented by Xiang et al. (2012) with stomatotypes of the Ab surface arranged accordingly.
Tetra, brachypara, latero represent tetracytic, brachyparacytic, and laterocytic, respectively.
It has been reported that the lateral wall of the meristemoid is never intersected by a transverse wall of the two adjacent files in the development of a stoma complex and the resultant stoma complex rarely occurs in the majority of the monocotyledons except Luisia [27]. It is interesting that Holcoglossum is also an exception with the anticlinal walls perpendicular to the long axis of the pore, which is found in its laterocytic and polarcytic types. The lateral wall of the meristemoid never intersected by a transverse wall of the two adjacent files has the value minimizing mechanical stresses in a meristem which enlarge chiefly in longitudinal direction [27]. We infer that the laterocytic and polarcytic types in Luisia and Holcoglossum are perhaps an adaptation to the longitudinal and intersected directions' growth of the tereted-leaves or semitereted-leaves. However, the percentage of laterocytic or polarcytic types is different between these two genera in this study, and it is much higher in Holcoglossum than in Luisia. The highest value is found in the AC clade of Holcoglossum, which is restricted to the Hengduan Mountains in southwestern China, and our results suggested that laterocytic or polarcytic is perhaps related to the cool winds and the heavy rains found in the high mountains [32].
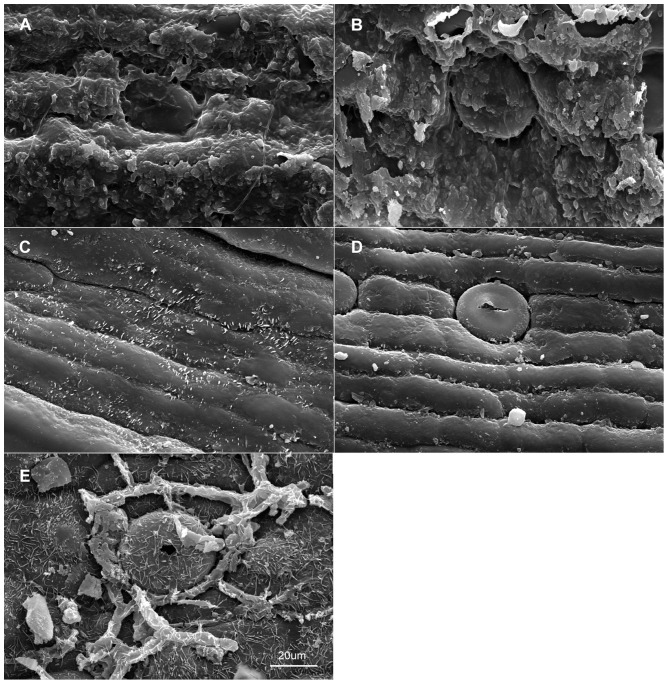
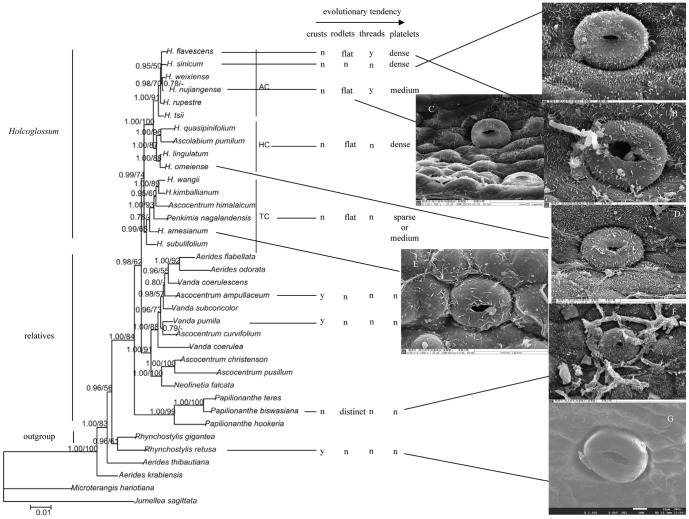
In the genus Holcoglossum, wax types on the Ab surface change and have a general tendency that is consistent with the tendency suggested by molecular phylogenetic studies [13], [20]. The rodlets vary from flat to threads or absent and the platelets vary from sparse or medium to dense in a direction of TC - HC - AC, (Fig. 11). A crust is only found in the outgroup and relatives of Holcoglossum; therefore, we suggest that small and dense platelets are more evolutionarily derived than crusts and rodlets (Fig. 11). The wax types vary in a direction of TC – HC – AC, and the latitude increases and the temperature decreases comparatively. Chemical analyses of the wax crystals might provide information that could explain the morphological differences in the wax crystals [35], [36].
Figure 11. Molecular phylogeny presented by Xiang et al. (2012) with wax types and SEMs of the Ab surface arranged accordingly.
A. Holcoglossum sinicum. B. H. flavescens. C. H. nujiangense. D. H. omeiense. E. H. amesianum. F. Papilionanthe biswasiana. G. Rhynchostylis retusa. y represents yes; n represents no.
Taxonomic suggestions for H. himalaicum
Holcoglossum himalaicum is a difficult species with different phylogenetic positions. Recent molecular phylogenetic studies suggest that it is a species of Holcoglossum [13] or Vanda [37]. In this study, nearly all of the characteristics of the leaf epidermis evaluated under LM are the same as those of the TC clade, including the phylogenetic characteristics for the infrageneric clades of Holcoglossum mentioned above (Tables 1 and 2). The wax types of H. himalaicum are the same as those of H. wangii. Therefore, H. himalaicum should be included in the TC clade of Holcoglossum.
Materials and Methods
Taxon sampling
The mature leaves of 27 herbarium specimens, representing 12 of 17 species of genus Holcoglossum [13], were used in this study (Table S1). An additional nine specimens, representing four species from the related genera Vanda, Papilionanthe, Luisia and Ascocentrum, were included for comparison with Holcoglossum (Table S1). Another two specimens of Rhynchostylis retusa were used as an outgroup, which is according to recent molecular phylogenetic studies [13], [20]–[23], [38] (Table S1). Samples were taken from herbarium specimens.
Methods
The materials for the LM study were boiled in water before maceration in 35% NaClO. Pieces of leaf epidermis were stained with safranin–alcohol (50%), and then dehydrated in an ethanol series before being mounted in Canada balsam. To verify the constancy of the epidermal structure, at least five slides were assembled from different parts of a single leaf for each sample. Because of the difficulty in controlling the time of maceration in 35% NaClO for all of the species of the AC clades, Rhynchostylis retusa and H. omeiense, only the wax coats with the prints of the epidermal cells were examined. In addition, certain slides of H. sinicum were mounted in water. The leaf samples for the SEM observation were taken from the midsection of the leaf to eliminate variation caused by sampling from different leaf areas. At least three accessions from two or more different collections for each species were sampled to assess the variation within a species. These samples were cut into small pieces ca. 0.5 cm+0.5 cm or ca. 0.5 cm in length, directly attached to stubs without any treatment. After gold sputtering, the samples were examined and photographed under a Hitachi JSM-35C, six samples from the species Rhynchostylis retusa; H. himalaicum; and Luisia magniflora were examined under a JSM-7001F.
Terminology
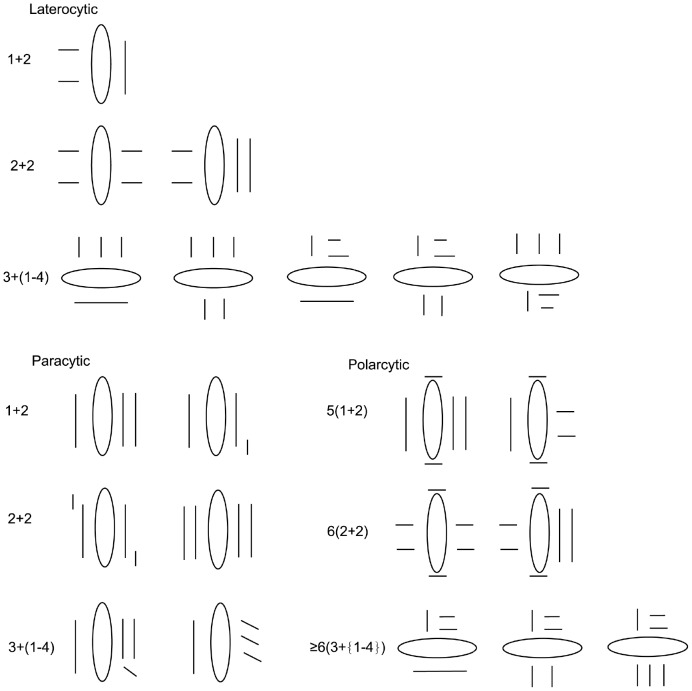
The stomatal terminology is consistent with that of Carpenter [39] because most of the stomatotypes present in this study, such as paracytic, laterocytic and stephanocytic, can be found in Carpenter's work. In addition, several stomatotypes were found with two short polar subsidiary cells and more than two subsidiary cells on at least one lateral side of the stoma, and these types are consistent with the examples from Williams [29], such as 6(4+2) (6 subsidiary cells, with 4 lateral and 2 polar). However, there are similar stomatal type names with different meanings in Carpenter [39], such as laterocytic 1+2 (3 subsidiary cells, with 1 on one lateral side and 2 on the other side). In this study, we named the 6(4+2) type from Williams the polarcytic 6(2+2) type, and it had 6 subsidiary cells, with 2 polar subsidiary cells (not included in the name) and 2 subsidiary cells on each lateral sides. In addition, we increase two subtypes of paracytic type: paracytic 1+2 and paracytic 3+(1–4), and they had the same meaning as the laterocytic subtypes in Carpenter [39]. Illustrations of these types are shown in Figure 12. The terminology for other characteristics was based on the classification of Wilkinson [40], and terminology of the epicuticular waxes was based on Barthlott et al. [35].
Figure 12. Subtypes of three stomatotypes commonly found in this study.
Notes: Every line represents a subsidiary cell and the orientation of its long axis. Its length represents its size, and an ellipse represents a stoma. The notation 1+2 of the laterocytic and paracytic types indicates the distribution of subsidiary cells; in this case, there is 1 on one lateral side of the stoma and 2 on the other side. The notation 5(1+2) of the polarcytic type indicates the distribution of subsidiary cells; in this case, there are five subsidiary cells, with 2 polar and 3 lateral subsidiary cells and 1 on one lateral side and 2 on the other side.
Supporting Information
Source of materials.
(DOC)
Acknowledgments
We thank Dr. W. Mark Whitte (Florida Museum of Natural History, University of Florida) for his critical comments and careful revision.
Funding Statement
The authors have no support or funding to report.
References
- 1.Dressler RL (1981) The orchids: natural history and classification: Harvard University Press, Cambridge.
- 2.Dressler RL (1993) Phylogeny and classification of the orchid family: Dioscorides, Portland.
- 3. Garay LA (1972) On the systematics of the monopodial orchids I. Bot Mus Leafl (Harvard University) 23: 149–212. [Google Scholar]
- 4. Garay LA (1974) On the systematics of the monopodial orchids II. Botanical Mus Leafl (Harvard University) 23: 369–376. [Google Scholar]
- 5. Seidenfaden G (1988) The orchid genera in Thailand XIV. Fifty-nine vandoid genera. Opera Bot 95: 304–308. [Google Scholar]
- 6. Seidenfaden G (1992) The orchids of Indochina. Opera Bot 114: 441–443. [Google Scholar]
- 7. Topik H, Yukawa T, Ito M (2005) Molecular phylogenetics of subtribe Aeridinae (Orchidaceae): insights from plastid matK and nuclear ribosomal ITS sequences. J Plant Res 118: 271–284. [DOI] [PubMed] [Google Scholar]
- 8.Christenson EA (1987) Taxonomy of the Aerides and related genera. In: Saito K, Tanaka R, eds. Proceedings of the 12th World Orchid Conference. Tokyo: Nippon Tosyo Tsushin Hanbai. 35–40.
- 9.Pridgeon AM, Cribb PJ, Chase MW, Rasmussen FN (2014) Genera Orchidacearum Volume 6: Epidendroideae (Part Three). Oxford: Oxford University Press.
- 10. Christenson EA (1987) An infrageneric classification of Holcoglossum Schltr. (Orchidaceae: Sarcanthinae) with a key to the genera of the Aerides-Vanda alliance. Notes Roy Bot Gard Edinburgh 44: 249–256. [Google Scholar]
- 11. Schlechter R (1919) Orchideologiae Sino-Japonicae Prodromus. Feddes Repert Spec Nov Regni Veg Beih 4: 285. [Google Scholar]
- 12. Jin XH (2005) Generic delimitation and a new infrageneric system in the genus Holcoglossum (Orchidaceae: Aeridinae). Bot J Linn Soc 149: 465–468. [Google Scholar]
- 13. Xiang XG, Li DZ, Jin XH, Hu H, Zhou HL, et al. (2012) Monophyly or paraphyly - the taxonomy of Holcoglossum (Aeridinae: Orchidaceae). PloS ONE 7: e52050 doi:10.1371/journal.pone.0052050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tsi ZH (1999) Aeridinae. In: Tsi ZH, ed. Flora Reipublicae Popularis Sinicae, vol. 19 . Beijing: Science Press. pp. 269–437. [Google Scholar]
- 15. Christenson EA (1998) Two new species of Holcoglossum Schltr. (Orichidaceae: Aeridinae) from China. Lindleyana 13: 121–124. [Google Scholar]
- 16. Jin XH, Qin HN, Chen SC (2004) A new species of Holcoglossum (Orchidaceae: Aeridinae) from China. Kew Bull 59: 633–635. [Google Scholar]
- 17. Jin XH, Chen SC, Qin HN, Zhu GH, Siu LP (2004) A new species of Holcoglossum (Orchidaceae) from China. Novon 14: 178–179. [Google Scholar]
- 18. Jin XH, Chen SC, Li DZ (2007) Holcoglossum nujiangense (Orchidaceae), a new species and its pollination system. Nord J Bot 25: 125–128. [Google Scholar]
- 19. Tsi ZH (1982) A study of the genus Holcoglossum of Orchidaceae. Acta Phytotaxonomica Sinica 20: 439–444. [Google Scholar]
- 20. Fan J, Qin HN, Li DZ, Jin XH (2009) Molecular phylogeny and biogeography of Holcoglossum (Orchidaceae: Aeridinae) based on nuclear ITS, and chloroplast trnL-F and matK. Taxon 3: 849–861. [Google Scholar]
- 21. Liu ZJ, Chen LJ, Chen SC, Cai J, Tsai WC, et al. (2011) Paraholcoglossum and Tsiorchis, two new orchid genera established by molecular and morphological analyses of the Holcoglossum alliance. PLoS ONE 6: e24864 doi:24810.21371/journal.pone.0024864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Baranova MA (1992) Principles of comparative stomatographic studies of flowering plants. Bot Rev 58: 49–99. [Google Scholar]
- 23. Carlsward BS, Whitten WM, Williams NH, Bytebier B (2006) Molecular phylogenetics of Vandeae (Orchidaceae) and the evolution of leaflessness. Am J Bot 93: 770–786. [DOI] [PubMed] [Google Scholar]
- 24. Baranova MA (1992) Principles of comparative stomatographic studies of flowering plants. Bot Rev 58: 49–99. [Google Scholar]
- 25. Yang Y, Zhang LY, Liu B, Werff H van der (2012) Leaf cuticular anatomy and taxonomy of Syndiclis (Lauraceae) and its allies. Systematic Botany 37 (4): 861–878. [Google Scholar]
- 26. Stern WL, Carlsward BS (2006) Comparative vegetative anatomy and systematics of the Oncidiinae (Maxillarieae, Orchidaceae). Bot J Linn Soc 152: 91–107. [Google Scholar]
- 27. Tomlinson PB (1974) Development of the stomatal complex as a taxonomic character in the Monocotyledons. Taxon 23: 109–128. [Google Scholar]
- 28. Carlsward BS, Stern WL, Bytebier B (2006) Comparative vegetative anatomy and systematics of the angraecoids (Vandeae, Orchidaceae) with an emphasis on the leafless habit. Bot J Linn Soc 151: 165–218. [Google Scholar]
- 29. Williams NH (1979) Subsidiary cells in the Orchidaceae: their general distribution with special reference to development in the Oncidieae. Bot J Linn Soc 78: 41–66. [Google Scholar]
- 30. Das S, Paria N (1992) Stomatal structure of some Indian orchids with reference to taxonomy. Bangladesh Journal of Botany 21: 65–72. [Google Scholar]
- 31. Wayne MM, Stern WL, Judd WS (1996) Vegetative anatomy and systematics of subtribe Dendrobiinae (Orchidaceae). Bot J Linn Soc 120: 89–144. [Google Scholar]
- 32.Kaushik P (1978) Ecological anatomy of some Himalayan Orchids. Ph. D. Thesis. Panjab Univ., Chandigarh, India.
- 33. Gan Y, Zhou L, Shen ZJ, Shen ZX, Zhang YQ, et al. (2010) Stomatal clustering, a new marker for environmental perception and adaptation in terrestrial plants. Bot Stud 51: 325–336. [Google Scholar]
- 34. Baranova M (1992) Principles of comparative stomatographic studies of flowering plants. Bot Rev 58: 1–99. [Google Scholar]
- 35. Barthlott W, Neinhuis C, Cutler D, Ditsch F, Meusel I, et al. (1998) Classification and terminology of plant epicuticular waxes. Bot J Linn Soc 126: 237–260. [Google Scholar]
- 36. Pansarin ER, Salatino A, Pereira AS (2008) Micromorphological and chemical characteristics of cuticular waxes of cleistes (Orchidaceae, Pogonieae). Bol. Bot. Univ. Sao Paulo 26(2): 79–91. [Google Scholar]
- 37. Gardiner LM (2012) New combinations in the genus Vanda (Orchidaceae). Phytotaxa 61: 47–54. [Google Scholar]
- 38. Gardiner LM, Kocyan A, Motes M, Roberts DL, Emerson BC (2013) Molecular phylogenetics of Vanda and related genera (Orchidaceae). Bot J Linn Soc 173: 549–572. [Google Scholar]
- 39. Carpenter KJ (2005) Stomal architecture and evolution in basal angiosperms. Am J Bot 92(10): 1595–1615. [DOI] [PubMed] [Google Scholar]
- 40.Wilkinson HP (1979) The plant surface (mainly leaf). In: Metcalfe CR, Chalk L, eds. Anatomy of the dicotyledons, 2nd edn. Oxford: Clarendon Press. 97–167.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Source of materials.
(DOC)