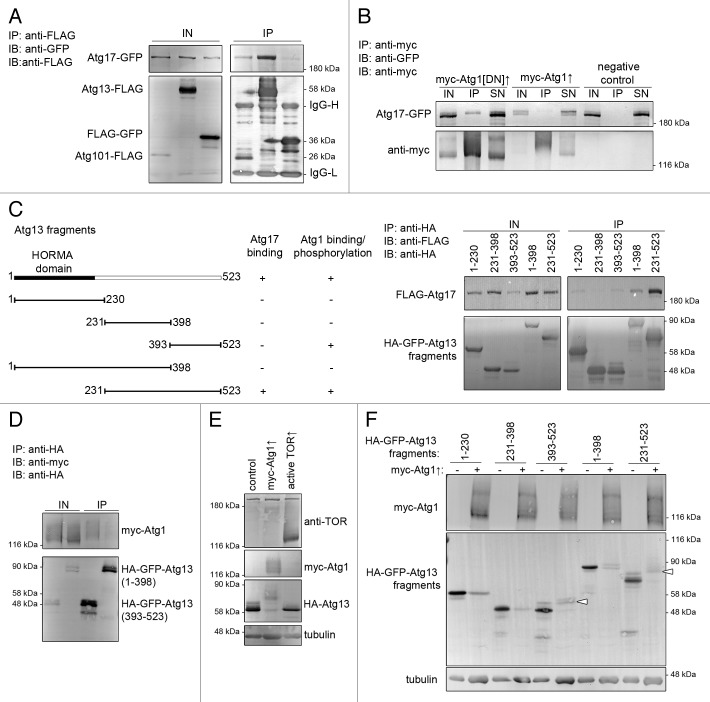
Figure 6. Interactions of Atg17, Atg1 and Atg13. (A and B) Immunoprecipitation experiments in cultured Drosophila cells. Atg17-GFP coprecipitates with Atg13-FLAG and Atg101-FLAG, but not with FLAG-GFP (A). Atg17-GFP binds weakly to kinase-dead and wild-type myc-Atg1, but not to anti-myc beads (B). Note the decreased mobility of Atg17-GFP upon coexpression of myc-Atg1. (C) Of the truncated Atg13 constructs (see left panel), a fragment containing the putative unstructured regions (231 to 523) binds strongly to Atg17-GFP. (D) Myc-Atg1 binds to the C-terminus (393 to 523) of Atg13. (E) Expression of myc-Atg1 strongly decreases and active TOR (amino acids 1333 to 2470) slightly increases the mobility of HA-Atg13 in cultured cells. (F) Expression of myc-Atg1 results in a shift (indicated by arrowheads) of Atg13 fragments containing the C terminus (393 to 523 and 231 to 523). IN, input, IP, immunoprecipitation, SN, supernatant.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.