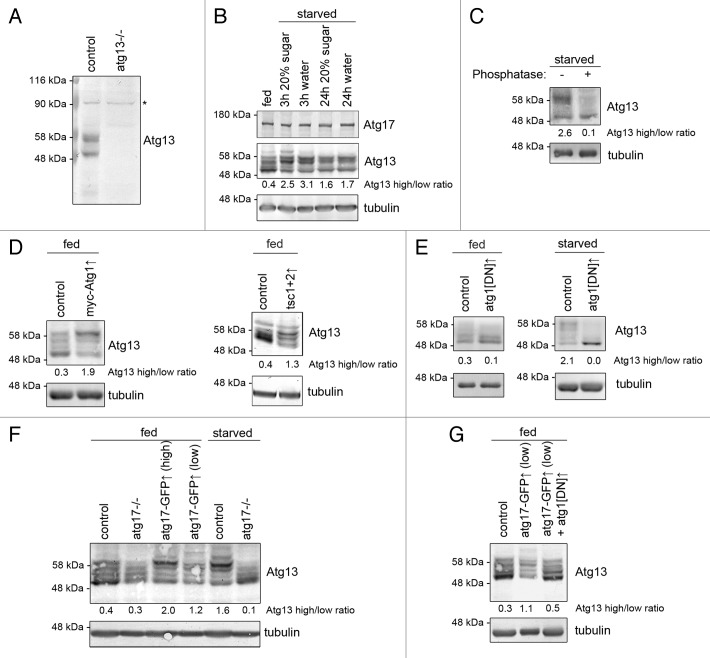
Figure 7. Atg17 regulates Atg13 phosphorylation in vivo. (A) Western blots using our novel antibody recognize endogenous Atg13, which is absent from atg13-null mutant larvae. Asterisk denotes a nonspecific band that serves as loading control. (B) Starvation strongly decreases the mobility of Atg13 in larval fat body extracts. (C) Phosphatase treatment of starved fat body lysates demonstrates that Atg13 is phosphorylated in vivo. (D) Transient overexpression of myc-Atg1, or Tsc1 and Tsc2 decreases Atg13 mobility in fat bodies of fed larvae. (E) Transient overexpression of dominant-negative Atg1 reduces the ratio of slower mobility Atg13 forms relative to higher mobility forms in fat bodies of well-fed larvae. Dominant-negative Atg1 expression practically eliminates all Atg13 phospho-forms in starved fat bodies. (F) Transient (low) or constitutive (high) expression of Atg17-GFP decreases Atg13 mobility in fat bodies of well-fed animals. The starvation-induced shift of Atg13 to slower mobility forms is lost in atg17-null mutants. (G) Coexpression of dominant-negative Atg1 prevents Atg17-induced shift of Atg13 to slower mobility forms in fat body extracts of well-fed larvae. Numbers indicating Atg13 high/low ratio are calculated by densitometric evaluation of blots as described in Materials and Methods.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.