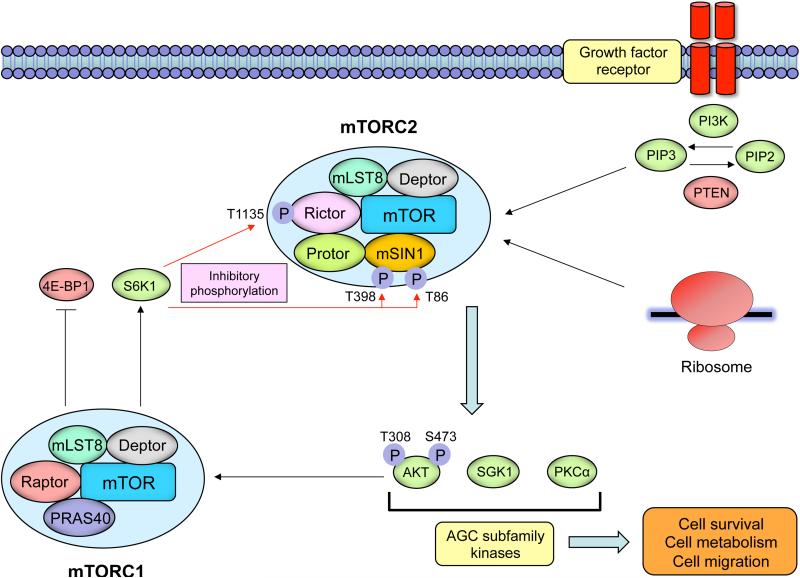
Figure 1. The structure and signaling of the mTORC2 complex.
Growth factor receptor-dependent activation of PI3K promotes mTORC2's binding to ribosomes, which activates mTORC2 in a fashion that is still incompletely understood. mTORC2 phosphorylates conserved motifs in AGC kinases to promote their allosteric activation. mTORC2 is a critical node in growth factor receptor-PI3K signaling, phosphorylating Akt on Ser473 to promote its maximal activation. mTORC2 is negatively regulated by mTORC1. S6K1 downstream of mTORC1 phosphorylates Rictor on Thr1135 and mSIN1 on Thr86 and Thr398, inhibiting mTORC2-dependent phosphorylation of AKT.