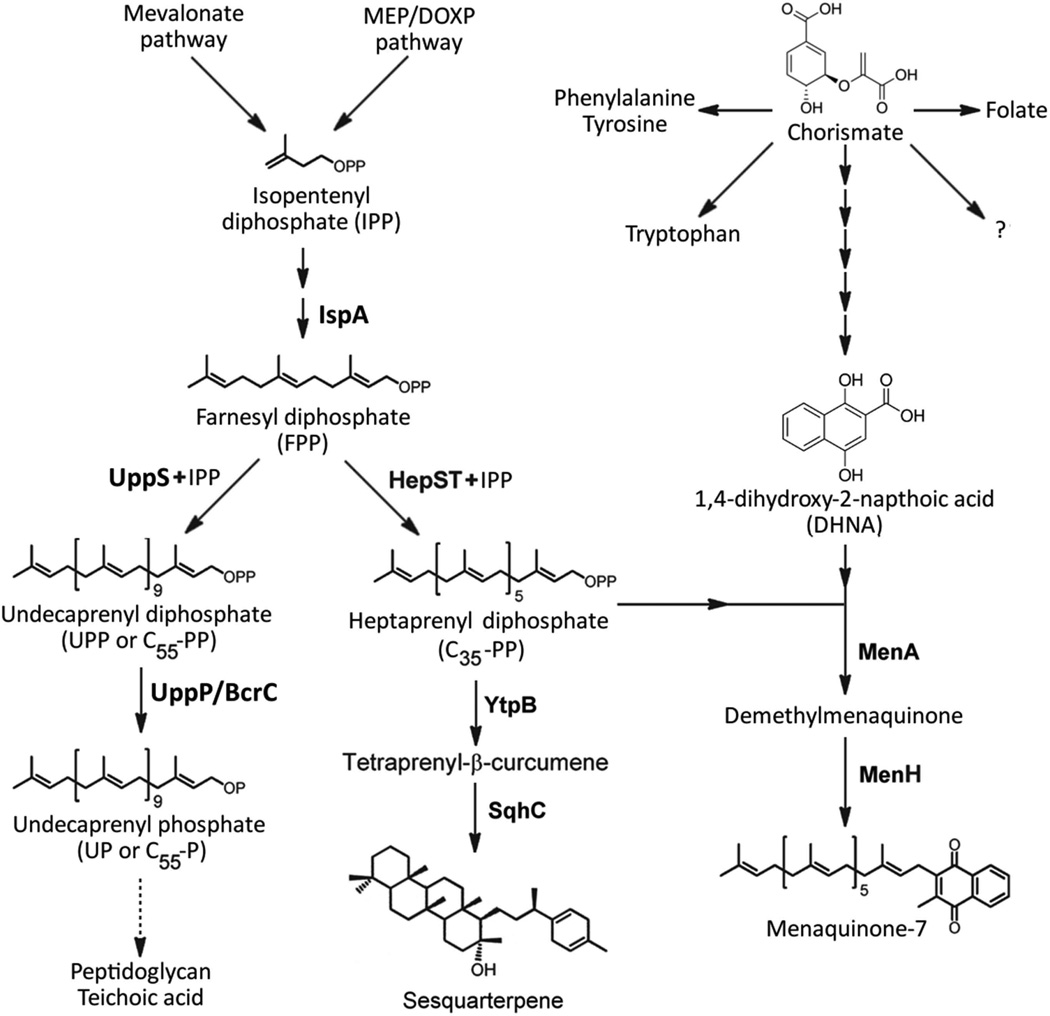
Figure 1.
Pathways of isoprenoid biosynthesis and utilization in Bacillus subtilis. Isopentenyl diphosphate is synthesized via the methylerythritol phosphate (MEP) pathway (Wagner et al., 2000). UppS adds eight consecutive isopentenyl diphosphate groups to the C15 farnesyl diphosphate (FPP) to generate undecaprenyl pyrophosphate (UPP=C55-PP), a key intermediate in the synthesis of cell wall components (Teng & Liang, 2012). The heterodimeric enzyme HepST also functions as a prenyltransferase and adds four isoprenyl units in head-to-tail condensation with FPP to generate the C35 intermediate, heptaprenyl diphosphate (C35-PP) (Zhang et al., 1998). The C35-PP intermediate is a substrate for YtpB, catalyzing the committed step in the synthesis of sesquarterpenes (Sato et al., 2011). The sesquarterpene shown has recently been named baciterpenol A (Sato, 2013). C35-PP is also a substrate for MenA, a central enzyme of the menaquinone-7 biosynthesis pathway (Suvarna et al., 1998). Depending on the growth conditions, MenA activity can be limited by the availability of the co-substrate 1,4-dihydroxy-2-naphthoic acid (DHNA) (see text for details), a metabolite derived from the central branch point intermediate chorismate.