Figure 5. Progenitor derived hepatocytes are not required for adaptation to oval cell injury in chimeric mice.
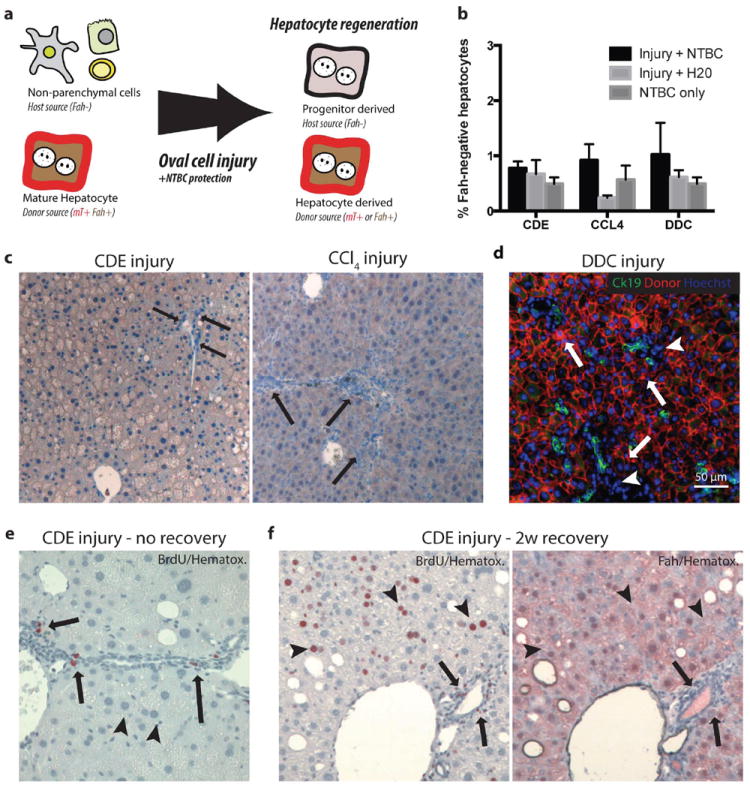
(a) Schematic: Hepatocyte transplantation into Fah-/- mice generated chimeric mice, host nonparenchymal cells were Fah- and unmarked. Donor hepatocytes were Fah+ and mTomato-marked. If nonparenchymal cells gave rise to hepatocytes after oval cell injury, we hypothesized that progenitor-derived Fah- hepatocytes would displace marked Fah+ hepatocytes. (b) Image quantification of Fah-negative hepatocytes demonstrates approximately 1% of hepatocytes remain host derived after injury. (c) No Fah-negative progenitor derived hepatocytes were observed at the majority portal regions (arrow = ductal proliferation) after 3 weeks CDE injury plus NTBC rescue or 10 weeks CCl4 injury plus NTBC (arrow = portal inflammation, ductal proliferation but no Fah- hepatocytes). (d) After 4 weeks DDC injury plus NTBC treatment CK19+ (green) ductal proliferations were adjacent to donor mTomato (red) hepatocytes (arrows) and inflammatory nonparenchmal cell (arrowhead, mTomato-negative) (bar=50μm). (e) BrdU uptake (2-hour pulse) was observed in proliferating ductal and nonparenchymal cells (arrow) but not hepatocytes at the peak of CDE injury. Hepatocytes show microvesicularsteatosis. (f) BrdU uptake (2 weeks BrdU water) during recovery from CDE injury was observed in hepatocytes (arrowheads), inflammatory cells, and cholangiocytes (arrows). Serial sectioning demonstrates that regenerating hepatocytes are lineage-marked mature hepatocytes (Fah+) and not derived from a nonparenchymal progenitor (Fah-).
