Figure 1. Spin trapping and fate of protein-DMPO adducts.
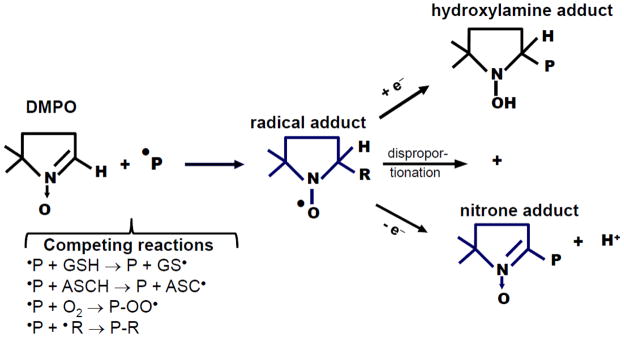
A protein radical (a radical site in a protein) reacts with DMPO to form a radical adduct. Depending on microenvironment conditions and structural characteristics of the target protein, the radical adduct can be reduced to hydroxylamine or oxidized to a stable nitrone adduct. It can also disproportionate to generate both the hydroxylamine and nitrone adducts. In cells and in vivo there are a number of competing reactions that can affect the yield of DMPO-protein adducts. Reduced glutathoine (GSH) and L-ascorbate (ASCH) [28] can react with protein radicals faster than the rate of reaction with DMPO, resulting in reduced yield of protein-DMPO nitrone adducts, a repaired protein and a less reactive radical (i.e., GS• and ASC•). Oxygen, the best spin trap in nature, can also react with protein radicals to form protein-peroxyl radicals, which very slowly react with DMPO. Protein radicals can react with other radical sites in the same or different proteins to form cross links (e.g., Tyr-Tyr, His-His or Trp-Trp). Protein radicals can also react with lipid radicals, •NO2/•NO or drug/toxicant radicals, thus resulting in protein-lipid or protein-drug/toxicant adducts (P-R).
