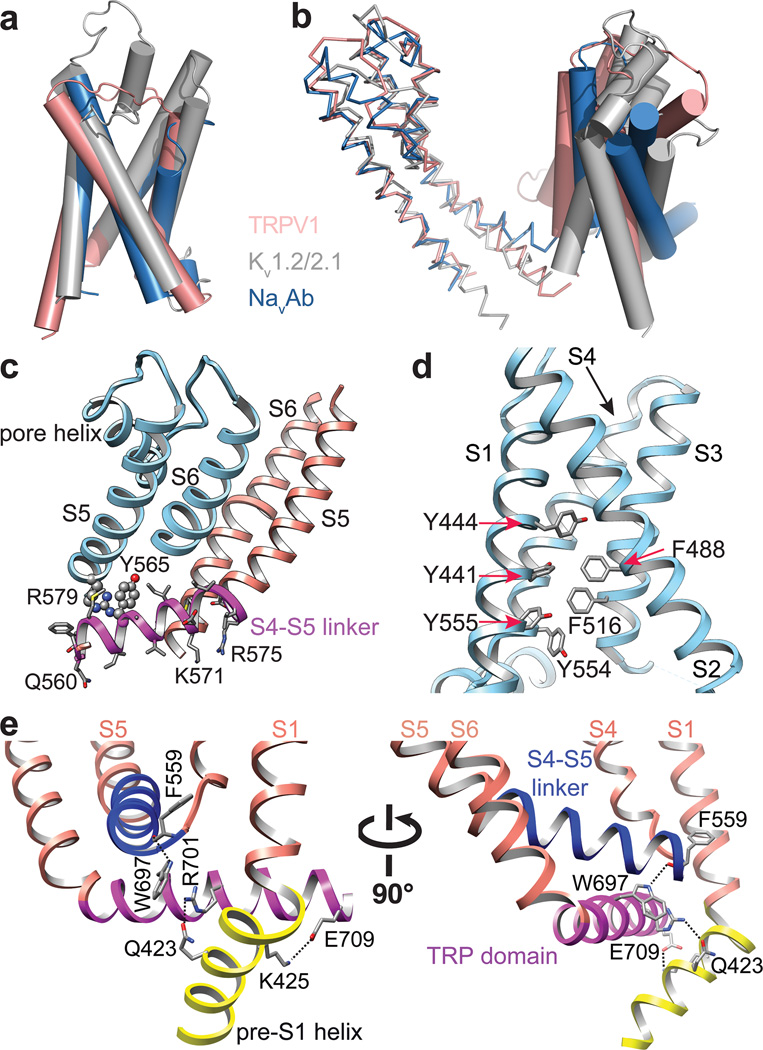
Figure 4. Unique structural features of TRPV1.
a, Alignment of S1–S4 transmembrane domains from TRPV1 (salmon), Kv1.2/2.1 chimera (PDB 2R9R; grey), and NavAb (PDB 3RVY; blue) show substantial overlap. b, When S5-P-S6 pore regions are aligned, the S1–S4 domains show differential relative orientations. c, The S4–S5 linker is an amphipathic α-helix whose charged surface faces the cytosol. Potential cation-π interactions between Y565 in the S4–S5 linker and R579 from S5 of the adjacent subunit are highlighted. Mutations of cognate residues in TRPV4 render the channel constitutively active and cause skeletal dysplasia. d, Aromatic side chains from S1, S3 and S4 helices create a hydrophobic interior in the S1–S4 domains, in contrast to the charged environment observed in VGICs. e, Two different views highlight interactions between TRP domain and S4–S5 linker and pre-S1 helix. Interactions (i.e. hydrogen bonds and salt bridge) are indicated by dashed lines.