Abstract
Although laparoscopic adrenalectomy has remained the standard of care for the treatment for adrenal tumors, percutaneous image-guided ablation therapy, such as chemical ablation, radiofrequency ablation, cryoablation, and microwave ablation, has been shown to be clinically useful in many nonsurgical candidates. Ablation therapy has been used to treat both functioning adenomas and malignant tumors, including primary adrenal carcinoma and metastasis. For patients with functioning adenomas, biochemical and symptomatic improvement is achieved in 96 to 100% after ablation; for patients with malignant adrenal neoplasms, however, the survival benefit from ablation therapy remains unclear, though good initial results have been reported. This article outlines the current role of ablation therapy for adrenal lesions, as well as identifying some of the technical considerations for this procedure.
Keywords: adrenal gland, radiofrequency ablation, cryoablation, microwave ablation, chemical ablation, interventional radiology
Objectives: Upon completion of this article, the reader will be able to describe the indications, outcomes, and complications associated with adrenal gland ablation procedures.
Accreditation: This activity has been planned and implemented in accordance with the Essential Areas and Policies of the Accreditation Council for Continuing Medical Education (ACCME) through the joint sponsorship of Tufts University School of Medicine (TUSM) and Thieme Medical Publishers, New York. TUSM is accredited by the ACCME to provide continuing medical education for physicians.
Credit: Tufts University School of Medicine designates this journal-based CME activity for a maximum of 1 AMA PRA Category 1 Credit™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
Adrenal neoplasms are a frequently encountered lesion, detected in 4 to 6% of patients undergoing diagnostic imaging studies.1 Surgical resection is considered the gold standard to treat most adrenal neoplasms. However, with the increased detection of incidental adrenal lesions due to the widespread use of medical imaging, and the need to treat patients with multiple comorbidities using less invasive techniques, there has been greater attention focused on percutaneous image-guided ablation for the treatment of adrenal tumors.1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 To date, there are multiple published reports describing the use of percutaneous ablation in the treatment of functioning adrenal tumors,1 2 3 4 5 6 7 8 9 10 11 as well as demonstrating its effectiveness in short-term local control of primary and metastatic adrenal neoplasms.1 2 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23
This review presents discussion of the indications for and techniques of image-guided ablation of adrenal neoplasms, as well as the outcomes and complications associated with such therapy.
Indication
Primary adrenal neoplasms, whether benign or malignant, are typically resected with open surgical and laparoscopic approaches.23 24 25 26 27 Surgery for local control has been controversial in patients with adrenal metastasis; however, selected patients with isolated metastatic disease to an adrenal gland have been reported to have a survival benefit from adrenalectomy.28 29 30 In those patients who are not surgical candidates either due to comorbid disease or who refuse surgery, image-guided radiofrequency ablation (RFA) is an alternative to surgery. In these situations, the indication for ablation therapy is similar to that of surgical intervention. As far as patient selection is concerned, tumor size is an important factor to achieve complete tumor necrosis, similar to performing percutaneous ablation in other organs.8 12 14 The smaller the maximum tumor diameter, the greater the likelihood of achieving local tumor control.8 12 14 Although the threshold size to expect therapeutic response has not been well evaluated, better results have been reported for adrenal tumors measuring 5 cm or smaller.12
Contraindications to adrenal ablative therapy are also similar to the contraindications of percutaneous ablation in other organs. Patients with an underlying coagulopathy should have this corrected before the ablation procedure; in general, the international normalized ratio (INR) should be less than 1.5, and the platelet count should be greater than 50,000/μL. The access route should be carefully evaluated to be sure that there is a safe approach, and specifically that there is no bowel that may be at risk for nontarget injury.
Preparation before Adrenal Ablation
Before ablation therapy, the tumor size, location, and approach route to the tumor should be evaluated using computed tomography (CT) and magnetic resonance imaging (MRI) with and without contrast enhancement. Planning the approach route is especially important for achieving effective tumor control and avoiding complications. As with any image-guided procedure, the risk of hemorrhage is significant. It is important to identify and consider any critical vascular structures that lie adjacent to the adrenal glands, including the inferior vena cava, aorta, renal arteries, and lumbar collateral vessels. Also, hypervascularity of the lesion itself can be a cause of hemorrhage. If the primary tumor (and subsequently the metastatic lesion) is hypervascular, such as hepatocellular carcinoma or renal cell carcinoma, combination therapy with adrenal arterial embolization and ablation therapy can be useful for preventing hemorrhagic complication and reinforcing antitumoral effects.14
In addition to careful access planning, some investigators suggest preprocedural and periprocedural adrenergic blockade to prevent hypertensive crisis.1 2 19 Hypertensive crisis is the result of a massive release of catecholamines stored by the gland that are released during the ablation of adrenal lesions22 23 24 31 32; this was confirmed in an animal model in which adrenal ablation was performed in swine.32 In this study, the swines' blood pressure increased to > 200 mm Hg in all six animals during adrenal ablation, with a significant increase in serum epinephrine and norepinephrine levels. In another study, Welch et al19 reported 13 adrenal cryoablation procedures in 12 patients with adrenal metastases. These authors reported hypertensive crisis in six procedures (46%, 6/13) during the thaw cycle.19 Of six patients who experienced hypertensive crisis, five had not received α-blockers before cryoablation.
Patient Position
Most adrenal tumors are accessible via a posterior approach with the patient in the prone position. However, some investigators suggest performing a posterior approach with the patient in an ipsilateral decubitus position1 2; this technique placed the targeted adrenal mass in a dependent position and also induces hypoinflation of the ipsilateral lung, reducing respiratory excursion of the diaphragm and reducing the chance of a transpleural path of the ablation probe that can result in pneumothorax or other lung injury during the ablation procedure.1 2
Nevertheless, difficulty can be encountered in approaching the targeted adrenal lesions with the patient in an ipsilateral decubitus position because this can result in the adrenal lesion abutting other critical organs, such as the pancreas or colon. In addition, the approach window becomes smaller in this position. Therefore, the authors usually place a patient in the prone position and evaluate the trajectory to ascertain whether the ablation probe can be placed safely into the adrenal lesion (Figs. 1 2 3). Tilting of the CT gantry is also a useful technique for avoiding a transpleural path of the ablation probe (Fig. 2). In instances where a safe approach cannot be identified with the patient prone, the authors will reposition the patient in an ipsilateral decubitus position and reimage.
Figure 1.
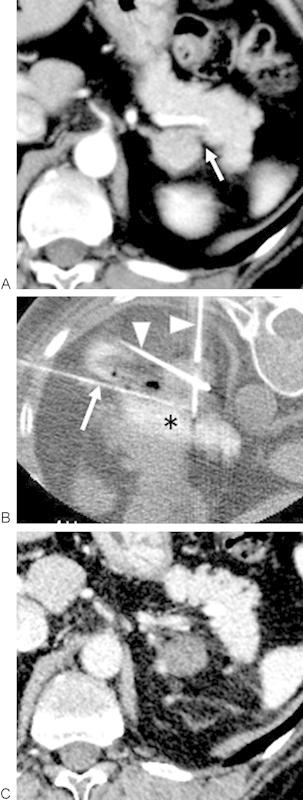
A 50-year-old woman who received radiofrequency ablation (RFA) of a functioning adenoma because of Cushing syndrome caused by a cortisol-secreting adenoma. (A) Contrast-enhanced axial CT images demonstrate an enhancing 2.4-cm maximum diameter tumor in the left adrenal gland. The adrenal tumor was adjacent to the pancreas (arrow). (B) Hydrodissection was performed after a 20-gauge needle was placed (white arrow) between the adrenal tumor and the pancreas. Water mixed with contrast medium (asterisk) was injected to displace the adrenal tumor from the pancreas. RF electrodes (arrowheads) were placed in the adrenal tumor, and the tumor was ablated in standard fashion. (C) Contrast-enhanced axial CT image obtained 3 days after RFA demonstrated lack of tumoral enhancement. Both serum cortisol and adrenocorticotropic hormone normalized, and the symptoms were alleviated. CT, computed tomography.
Figure 2.
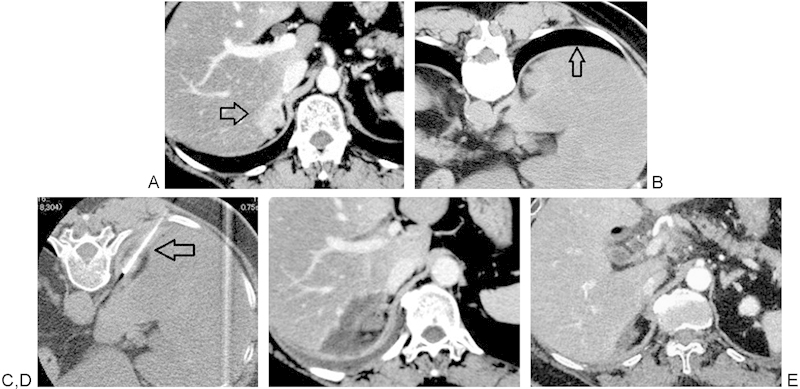
A 58-year-old woman who received RFA because of local recurrence of adrenal cortical cancer 6 years following right adrenalectomy. (A) Contrast-enhanced axial CT image demonstrated a recurrent, 3.6-cm maximum diameter tumor in the adrenal bed invading the liver (arrow). (B) Aerated lung is visible (arrow) behind the recurrent tumor on a CT image obtained with the patient in prone position. (C) The CT gantry was angled to avoid a transpleural path of the RF electrode. An RF electrode was placed in the tumor (arrow) without traversing the pleura. (D) Contrast-enhanced axial CT image demonstrates resolution of tumoral enhancement 1 week following RFA. (E) Recurrent tumor resolved on 3 years follow-up scan. CT, computed tomography; RFA, radiofrequency ablation.
Figure 3.
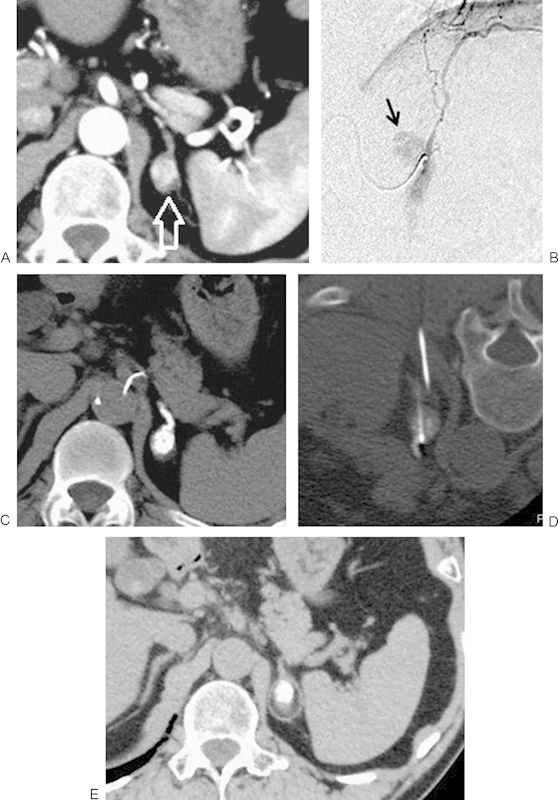
An 82-year-old man who received RFA because of adrenal metastasis from renal cell carcinoma 9 years following left nephrectomy. (A) Contrast-enhanced CT image demonstrates a hypervascular tumor in the left adrenal gland (arrow). (B) Left adrenal artery from the left inferior phrenic artery was embolized using iodized oil and gelatin sponge before RFA. Tumor enhancement was seen (arrow). (C) CT images acquired immediately after embolization demonstrates an iodized-oil deposit in the adrenal tumor. (D) RFA was performed after embolization. (E) Axial CT image obtained 2 years following RFA demonstrates adrenal tumor shrinkage. CT, computed tomography; RFA, radiofrequency ablation.
Image Guidance
CT or CT fluoroscopy is a widely available technique that enables visualization of the targeted adrenal mass, the path of the ablation probe, and the position of nearby critical anatomic structures such as kidney and colon; these factors make CT the preferred imaging modality for performing adrenal ablation.
Ultrasound is used less frequently because it is often difficult to identify adrenal glands by ultrasound. Moreover, assessment of tumor coverage by thermal ablation can be challenging because of either increased echogenicity of the ablation area from thermal destruction and gas or shadowing by the cryoablation ice ball.
Although MRI guidance has been used in the treatment of renal tumors with cryoablation, their usefulness in adrenal ablation therapy has not yet been established.33 34
Ablation Modality
Radiofrequency Ablation
The safety and efficacy of RFA is well established in the treatment of liver, lung, and renal tumors. Using alternating electrical current with frequencies of less than 30 MHz (usually 375–500 kHz) to generate heat, RFA induces thermal damage and tissue necrosis in the tissue adjacent to the electrode tip.1 2 4 5 6 7 8 10 11 12 14 15 Tissue destruction is achieved once the temperature threshold for cell death (50–60°C) has been reached.
The benefits of RFA include a well-established safety profile, widespread availability, and predictable thermal volumes based on the probe type. The procedure involves placement of a single or multiple probes into the tumor under imaging guidance. A grounding pad (typically placed on the patient's upper thigh) creates a closed-loop circuit for the current to travel.
Microwave Ablation
Microwave ablation works by using electromagnetic energy with frequencies of 30 MHz to 30 GHz to agitate water molecules, causing frictional heating and cell death by coagulative necrosis.1 2 10 18 The benefits of microwave ablation compared with RFA are a more rapid increase in temperature, the ability to achieve higher local temperatures, larger ablation volumes in a shorter ablation times, and the obviation of the need for grounding pads.
Cryoablation
Cryoablation causes cell death by the application of alternating cycles of freezing and thawing. Expansion of argon forced through a small internal aperture in the probe reaches very low temperatures (–80 to –150°C) by the Joule–Thomson effect.1 2 19 Active thawing is achieved by the application of helium. The alternating cycles of freezing and thawing cause mechanical stress on the cellular membranes from intracellular ice crystal formation, hypotonic cell disruption, and microvascular thrombosis.
The advantage of cryoablation is visualization of the ice ball during treatment with either CT or MR imaging. This enables one to monitor the area of thermal injury, thereby avoiding damage to adjacent organs. Cryoablation also causes less periprocedural pain than RFA. The limitations of cryoablation are the increased risk of hemorrhage caused by microvascular thrombosis and the inability to coagulate tissue during probe withdrawal, such as can be done with RFA and microwave ablation.
Chemical Ablation
Chemical ablation of the adrenal gland can be performed using imaging-guided percutaneous direct injection of either ethanol or acetic acid into the adrenal tumor. This is typically performed using several small (19–22 gauge) needles placed under CT, real-time CT fluoroscopy, or ultrasound guidance.1 2 10 18 Chemical ablation works by protein denaturation that causes coagulative necrosis and thrombosis of small vessels.
An important benefit of chemical ablation is that there is much less risk for collateral damage to adjacent organs. The downside to chemical ablation is the small ablative zone obtained during one treatment session, necessitating more frequent treatment sessions reported than for RFA.7 35
Hydrodissection
Adjacent organs may be in close proximity or abut the adrenal lesion being targeted. In these situations, hydrodissection can allow for safe treatment while concurrently protecting the adjacent structure. Hydrodissection is the instillation of fluid (typically a nonionic solution, such as sterile D5W) into the space between the adrenal lesion and the other critical structure (such as the pancreas, duodenum, colon, kidneys, and liver), thereby protecting these organs from thermal injury.36 37 Hydrodissection is generally performed by the insertion of an 18- to 20-gauge needle into the space between the adrenal gland and the critical organ, followed by the slow injection of fluid into the space (Fig. 1). Organ displacement is confirmed using imaging before and during the ablation to ensure there is appropriate margin between the nontarget organ and the ablation probe.
Outcomes
Functioning Adenoma
The use of RFA and chemical ablation has been reported in the treatment of functioning adenomas, including aldosteronoma, cortisol-secreting adenoma, and pheochromocytoma.5 6 7 9 In this population, good local tumor control rates have been reported irrespective of ablation modalities (Table 1). Based on enhancement patterns, adenomas are completely treated in 75 to 100% of cases after a single ablation, and 100% of cases after a second ablation session.5 6 7 9 It is important to keep in mind, though, that the ultimate aim is the correction of abnormal hormone release. Arima et al reported the relation between local tumor control and resolution of biochemical markers.7 In this study, four patients with cortisol-secreting adenomas were treated with RFA. There was no tumor enhancement in three of four patients after the initial RF session, leading to normalization of serum cortisol and adrenocorticotropic hormone (ACTH) levels and concurrent improvement in symptoms. Crescentic tumoral enhancement remained at the periphery of the adrenal tumor in the fourth patient, and although serum cortisol level had normalized the serum ACTH level remained abnormal in this patient. After a second adrenal RFA, there was no further lesion enhancement, and the cortisol and ACTH levels returned to normal.
Table 1. Outcomes in patients with functioning adenomas after ablation therapy.
| Author | Year | Type of ablation | Tumor no. | Patient no. | Type of functioning tumor | Tumor size (cm) | Resolution of Biochemical marker | Follow-up | Residual or recurrence | Complication |
|---|---|---|---|---|---|---|---|---|---|---|
| Arima et al7 | 2007 | RFA | 4 | 4 | Cortisol-secreting adenoma | 2.7 ± 0.6 (2.0–3.5) | All patients (100%) | 33 mo (20–46 mo) | 25% (¼) after first ablation, 0% after second ablation | Pneumothorax (n = 1) |
| Xiao et al9 | 2008 | PAI, PEI | 15 | 12 | Aldosteronoma (n = 11), cortisol-secreting adenoma (n = 6) | 2.8 ± 0.7 (2.1–4.4) | All patients (100%) | 2 y | 0% | No |
| Mendiratta-Lala et al5 | 2010 | RFA | 13 | 13 | Aldosteronoma (n = 10), cortisol-secreting adenoma (n = 1), testosterone-secreting adenoma (n = 1), pheochromocytoma (n = 1) | < 3.2 (1.0–3.2) | All patients (100%) | 21.2 mo (6–60 mo) | 0% | Small pneumothorax (n = 1) Limited hemothorax (n = 1) Self-limited procedural hypertension (n = 2) |
| Liu et al6 | 2010 | RFA | 24 | 24 | Aldosteronoma | 1.6 (0.4–2.5) | 23 of 24 patients (95.8%) | 21.2 mo (6.1–38.5 mo) | 0% | Small pneumothorax (n = 1) Retroperitoneal hematoma (n = 3) |
Abbreviations: mo, months; PAI, percutaneous acetic acid injection; PEI, percutaneous ethanol injection; RFA, radiofrequency ablation.
Malignant Adrenal Tumors
RFA, cryoablation, microwave ablation, ethanol injection, and acetic acid injection have been reported in the treatment of malignant adrenal tumors, including both adrenocortical carcinoma and adrenal metastases (Table 2). The three most frequent metastases that have been described as treated with adrenal ablation were lung cancer, hepatocellular carcinoma, and renal cell carcinoma. Using thermal ablation, the reported rates of residual or recurrent disease in treating adrenal malignancies are as low as 0 to 25%, during a mean follow-up of 10.3 to 37.7 months.8 9 10 12 14 17 18 19 On the contrary, chemical ablation using ethanol or acetic acid injection appears to be less efficacious for controlling adrenal metastases. Xiao et al treated 20 adrenal metastases using ethanol or acetic acid injection and found residual or recurrent disease in 14 tumors (70%) during the follow-up period of 20 months.9 In a separate study, Shibata et al treated nine adrenal metastases from hepatocellular carcinoma by ethanol injection and found residual or recurrent tumors in three tumors (33%) during the follow-up of 19.3 months.17
Table 2. Outcomes in patients with malignant adrenal tumors after ablation therapy.
| Author | Year | Type of ablation | Tumor no. | Patient no. | Type of tumor | Tumor size | Follow-up | Local residual or recurrence rate | Complication | Prognosis |
|---|---|---|---|---|---|---|---|---|---|---|
| Shibata et al17 | 2000 | PEI | 9 | 7 | Metastasis from HCC | 3.8 cm (2.5–6.0 cm) | 19.3 mo (6–36 mo) | 33% (3/9) | Adrenal insufficiency (n = 1) | 3 patients (42.9%) died at 8–36 mo 4 patients (57.1%) are alive at 6–28 mo |
| Wood et al12 | 2003 | RFA | 15 | 8 | Primary adrenal ca. and adrenal ca. metastasis to other sites | 4.3 cm (1.5–9.0 cm) | 10.3 mo (1–20 mo) | 20% (3/15) | No | NA |
| Mayo-Smith and Dupuy8 | 2004 | RFA | 11 | 10 | Metastasis from lung ca. (n = 5), RCC (n = 4), melanoma (n = 2) | 3.9 cm (1–8 cm) | 11.2 mo (1–46 mo) | 18.2% (2/11) | Small hematoma (n = 1) Adrenal insufficiency (n = 1) | 6 of 10 patients (60%) died at 3–16 months |
| Xiao et al9 | 2008 | ACI, PEI | 20 | 14 | Metastasis from lung ca. (n = 9), liver ca (n = 6), gastric ca. (n = 3), RCC (n = 2) | 5.9 cm (3.1–8.6 cm) | 24 mo | 70% (14/20) | No | 5 of 14 patients (35.7%) died at 2 years |
| Welch et al19 | 2011 | CA | 12 | 12 | Metastasis from RCC (n = 6), HCC (n = 1), lung ca. (n = 2), others (n = 3) | 2.7 cm (1.2–4.5 cm) | 18 mo (3–55 mo) | 9.1% (1/11) | Hypertensive crisis (n = 6) | NA |
| Yamakado et al14 | 2009 | TACE + RFA | 8 | 6 | Metastasis from HCC | 5.2 ± 1.8 cm (3.5–8.0 cm) | 37.7 mo (4.0–70.9 mo) | 25% (2/8) | No | Median survival time, 24.9 months |
| Li et al18 | 2011 | MW | 10 | 9 | Primary adrenal ca. (n = 1), adrenal metastasis (n = 9) | 3.8 cm (2.1–6.1 cm) | 11.3 (3–37) | 10% after first ablation, 0% after second ablation | Hypertensive crisis (n = 1) | NA |
| Wolf et al10 | 2012 | RFA, MW | 20 | 19 | Metastasis from RCC (n = 7), lung ca. (n = 8), HCC (n = 1), others (n = 4) | 4.2 cm (2–8 cm) | 14.1 mo (1–67 mo) | 15% after first ablation, 5% after reablation | Hypertensive crisis (n = 2) | 16 of 19 patients (84.2%) died during follow-up |
Abbreviations: CA, cryoablation; HCC, hepatocellular carcinoma; MW, microwave ablation; mo, months; NA, not available; PAI, percutaneous acetic acid injection; PEI, percutaneous ethanol injection; RFA, radiofrequency ablation; RCC, renal cell carcinoma; TACE, transarterial chemoembolization.
Complications
Several possible complications specific to adrenal ablation have already been discussed, including hypertensive crisis and thermal injury to adjacent organs.
Other potential complications include bleeding, infection, tumor seeding of the ablation probe tract, and pneumothorax (Tables 1 and 2). Adrenal insufficiency might occur if the contralateral adrenal gland has been removed, or if bilateral adrenal glands are treated.8 17
Survival Benefit
Few studies have evaluated the survival benefit of adrenal ablation because of inhomogeneous patient series, small patient numbers, and short-term follow-up.8 9 10 12 14 17 18 19 For tumors that are metastatic to the adrenal gland, the eventual outcome depends on the primary tumor prognosis. Yamakado et al reported the possibility of providing a survival benefit to selected patients with adrenal metastases.14 These authors treated adrenal metastases from hepatocellular carcinoma in six patients by adrenal chemoembolization and RFA, and reported long survival of more than 4 years in three patients treated for both intrahepatic and extrahepatic lesions.
Conclusion
Image-guided RFA of the adrenal glands is an effective procedure for the local control of primary or metastatic adrenal malignancy and as an alternative minimally invasive therapy for functioning adrenal tumors. Long-term outcomes, however, must be clarified.
References
- 1.Ethier M D, Beland M D, Mayo-Smith W. Image-guided ablation of adrenal tumors. Tech Vasc Interv Radiol. 2013;16(4):262–268. doi: 10.1053/j.tvir.2013.08.008. [DOI] [PubMed] [Google Scholar]
- 2.Uppot R N, Gervais D A. Imaging-guided adrenal tumor ablation. AJR Am J Roentgenol. 2013;200(6):1226–1233. doi: 10.2214/AJR.12.10328. [DOI] [PubMed] [Google Scholar]
- 3.Liang H L, Pan H B, Lee Y H. et al. Small functional adrenal cortical adenoma: treatment with CT-guided percutaneous acetic acid injection—report of three cases. Radiology. 1999;213(2):612–615. doi: 10.1148/radiology.213.2.r99nv10612. [DOI] [PubMed] [Google Scholar]
- 4.Al-Shaikh A A, Al-Rawas M M, Al-Asnag M A. Primary hyperaldosteronism treated by radiofrequency ablation. Saudi Med J. 2004;25(11):1711–1714. [PubMed] [Google Scholar]
- 5.Mendiratta-Lala M, Brennan D D, Brook O R. et al. Efficacy of radiofrequency ablation in the treatment of small functional adrenal neoplasms. Radiology. 2011;258(1):308–316. doi: 10.1148/radiol.10100690. [DOI] [PubMed] [Google Scholar]
- 6.Liu S Y, Ng E K, Lee P S. et al. Radiofrequency ablation for benign aldosterone-producing adenoma: a scarless technique to an old disease. Ann Surg. 2010;252(6):1058–1064. doi: 10.1097/SLA.0b013e318f66936. [DOI] [PubMed] [Google Scholar]
- 7.Arima K, Yamakado K, Suzuki R. et al. Image-guided radiofrequency ablation for adrenocortical adenoma with Cushing syndrome: outcomes after mean follow-up of 33 months. Urology. 2007;70(3):407–411. doi: 10.1016/j.urology.2007.04.032. [DOI] [PubMed] [Google Scholar]
- 8.Mayo-Smith W W, Dupuy D E. Adrenal neoplasms: CT-guided radiofrequency ablation—preliminary results. Radiology. 2004;231(1):225–230. doi: 10.1148/radiol.2311031007. [DOI] [PubMed] [Google Scholar]
- 9.Xiao Y Y, Tian J L, Li J K, Yang L, Zhang J S. CT-guided percutaneous chemical ablation of adrenal neoplasms. AJR Am J Roentgenol. 2008;190(1):105–110. doi: 10.2214/AJR.07.2145. [DOI] [PubMed] [Google Scholar]
- 10.Wolf F J, Dupuy D E, Machan J T, Mayo-Smith W W. Adrenal neoplasms: effectiveness and safety of CT-guided ablation of 23 tumors in 22 patients. Eur J Radiol. 2012;81(8):1717–1723. doi: 10.1016/j.ejrad.2011.04.054. [DOI] [PubMed] [Google Scholar]
- 11.Yamakado K, Takaki H, Yamada T. et al. Incidence and cause of hypertension during adrenal radiofrequency ablation. Cardiovasc Intervent Radiol. 2012;35(6):1422–1427. doi: 10.1007/s00270-012-0348-6. [DOI] [PubMed] [Google Scholar]
- 12.Wood B J, Abraham J, Hvizda J L, Alexander H R, Fojo T. Radiofrequency ablation of adrenal tumors and adrenocortical carcinoma metastases. Cancer. 2003;97(3):554–560. doi: 10.1002/cncr.11084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Atwell T D, Wass C T, Charboneau J W, Callstrom M R, Farrell M A, Sengupta S. Malignant hypertension during cryoablation of an adrenal gland tumor. J Vasc Interv Radiol. 2006;17(3):573–575. doi: 10.1097/01.RVI.0000197370.83569.33. [DOI] [PubMed] [Google Scholar]
- 14.Yamakado K, Anai H, Takaki H. et al. Adrenal metastasis from hepatocellular carcinoma: radiofrequency ablation combined with adrenal arterial chemoembolization in six patients. AJR Am J Roentgenol. 2009;192(6):W300–W305. doi: 10.2214/AJR.08.1752. [DOI] [PubMed] [Google Scholar]
- 15.Carrafiello G, Laganà D, Recaldini C. et al. Imaging-guided percutaneous radiofrequency ablation of adrenal metastases: preliminary results at a single institution with a single device. Cardiovasc Intervent Radiol. 2008;31(4):762–767. doi: 10.1007/s00270-008-9337-1. [DOI] [PubMed] [Google Scholar]
- 16.Wang Y, Liang P, Yu X, Cheng Z, Yu J, Dong J. Ultrasound-guided percutaneous microwave ablation of adrenal metastasis: preliminary results. Int J Hyperthermia. 2009;25(6):455–461. doi: 10.1080/02656730903066608. [DOI] [PubMed] [Google Scholar]
- 17.Shibata T, Maetani Y, Ametani F, Itoh K, Konishi J. Percutaneous ethanol injection for treatment of adrenal metastasis from hepatocellular carcinoma. AJR Am J Roentgenol. 2000;174(2):333–335. doi: 10.2214/ajr.174.2.1740333. [DOI] [PubMed] [Google Scholar]
- 18.Li X, Fan W, Zhang L. et al. CT-guided percutaneous microwave ablation of adrenal malignant carcinoma: preliminary results. Cancer. 2011;117(22):5182–5188. doi: 10.1002/cncr.26128. [DOI] [PubMed] [Google Scholar]
- 19.Welch B T, Atwell T D, Nichols D A. et al. Percutaneous image-guided adrenal cryoablation: procedural considerations and technical success. Radiology. 2011;258(1):301–307. doi: 10.1148/radiol.10100631. [DOI] [PubMed] [Google Scholar]
- 20.Venkatesan A M, Locklin J, Lai E W. et al. Radiofrequency ablation of metastatic pheochromocytoma. J Vasc Interv Radiol. 2009;20(11):1483–1490. doi: 10.1016/j.jvir.2009.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tsoumakidou G, Buy X, Zickler P, Zupan M, Douchet M P, Gangi A. Life-threatening complication during percutaneous ablation of adrenal gland metastasis: Takotsubo syndrome. Cardiovasc Intervent Radiol. 2010;33(3):646–649. doi: 10.1007/s00270-009-9612-9. [DOI] [PubMed] [Google Scholar]
- 22.Chini E N, Brown M J, Farrell M A, Charboneau J W. Hypertensive crisis in a patient undergoing percutaneous radiofrequency ablation of an adrenal mass under general anesthesia. Anesth Analg. 2004;99(6):1867–1869. doi: 10.1213/01.ANE.0000136803.54212.E1. [DOI] [PubMed] [Google Scholar]
- 23.Lo W K, vansonnenberg E, Shankar S. et al. Percutaneous CT-guided radiofrequency ablation of symptomatic bilateral adrenal metastases in a single session. J Vasc Interv Radiol. 2006;17(1):175–179. doi: 10.1097/01.rvi.0000188748.51764.ce. [DOI] [PubMed] [Google Scholar]
- 24.Onik G, Onik C, Medary I. et al. Life-threatening hypertensive crises in two patients undergoing hepatic radiofrequency ablation. AJR Am J Roentgenol. 2003;181(2):495–497. doi: 10.2214/ajr.181.2.1810495. [DOI] [PubMed] [Google Scholar]
- 25.Demeure M J, Somberg L B. Functioning and nonfunctioning adrenocortical carcinoma: clinical presentation and therapeutic strategies. Surg Oncol Clin N Am. 1998;7(4):791–805. [PubMed] [Google Scholar]
- 26.Pommier R F Brennan M F An eleven-year experience with adrenocortical carcinoma Surgery 19921126963–970., discussion 970–971 [PubMed] [Google Scholar]
- 27.Sung G T, Gill I S. Laparoscopic adrenalectomy. Semin Laparosc Surg. 2000;7(3):211–222. [PubMed] [Google Scholar]
- 28.Paul C A, Virgo K S, Wade T P, Audisio R A, Johnson F E. Adrenalectomy for isolated adrenal metastases from non-adrenal cancer. Int J Oncol. 2000;17(1):181–187. [PubMed] [Google Scholar]
- 29.Lo C Y, van Heerden J A, Soreide J A. et al. Adrenalectomy for metastatic disease to the adrenal glands. Br J Surg. 1996;83(4):528–531. doi: 10.1002/bjs.1800830432. [DOI] [PubMed] [Google Scholar]
- 30.Kim S H, Brennan M F, Russo P, Burt M E, Coit D G. The role of surgery in the treatment of clinically isolated adrenal metastasis. Cancer. 1998;82(2):389–394. [PubMed] [Google Scholar]
- 31.Yamakado K, Nakatsuka A, Takaki H. et al. Early-stage hepatocellular carcinoma: radiofrequency ablation combined with chemoembolization versus hepatectomy. Radiology. 2008;247(1):260–266. doi: 10.1148/radiol.2471070818. [DOI] [PubMed] [Google Scholar]
- 32.Yamakado K, Takaki H, Uchida K, Nakatsuka A, Shiraishi T, Takeda K. Adrenal radiofrequency ablation in swine: change in blood pressure and histopathologic analysis. Cardiovasc Intervent Radiol. 2011;34(4):839–844. doi: 10.1007/s00270-010-0078-6. [DOI] [PubMed] [Google Scholar]
- 33.Tatli S, Morrison P R, Tuncali K, Silverman S G. Interventional MRI for oncologic applications. Tech Vasc Interv Radiol. 2007;10(2):159–170. doi: 10.1053/j.tvir.2007.09.008. [DOI] [PubMed] [Google Scholar]
- 34.Goldberg S N Grassi C J Cardella J F et al. Image-guided tumor ablation: standardization of terminology and reporting criteria J Vasc Interv Radiol 200920(7, Suppl):S377–S390. [DOI] [PubMed] [Google Scholar]
- 35.Livraghi T, Goldberg S N, Lazzaroni S, Meloni F, Solbiati L, Gazelle G S. Small hepatocellular carcinoma: treatment with radio-frequency ablation versus ethanol injection. Radiology. 1999;210(3):655–661. doi: 10.1148/radiology.210.3.r99fe40655. [DOI] [PubMed] [Google Scholar]
- 36.Farrell M A, Charboneau J W, Callstrom M R, Reading C C, Engen D E, Blute M L. Paranephric water instillation: a technique to prevent bowel injury during percutaneous renal radiofrequency ablation. AJR Am J Roentgenol. 2003;181(5):1315–1317. doi: 10.2214/ajr.181.5.1811315. [DOI] [PubMed] [Google Scholar]
- 37.Takaki H, Nakatsuka A, Uraki J. et al. Renal cell carcinoma: radiofrequency ablation with a multiple-electrode switching system—a phase II clinical study. Radiology. 2013;267(1):285–292. doi: 10.1148/radiol.12121070. [DOI] [PubMed] [Google Scholar]


