Figure 2.
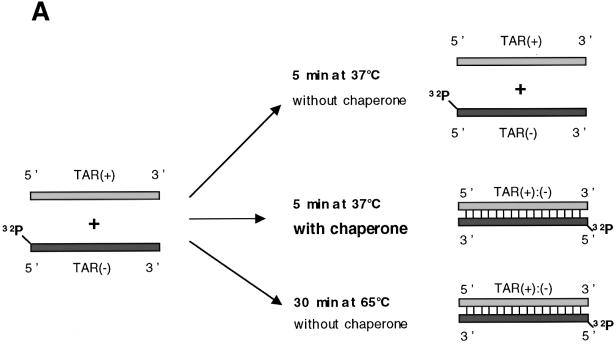
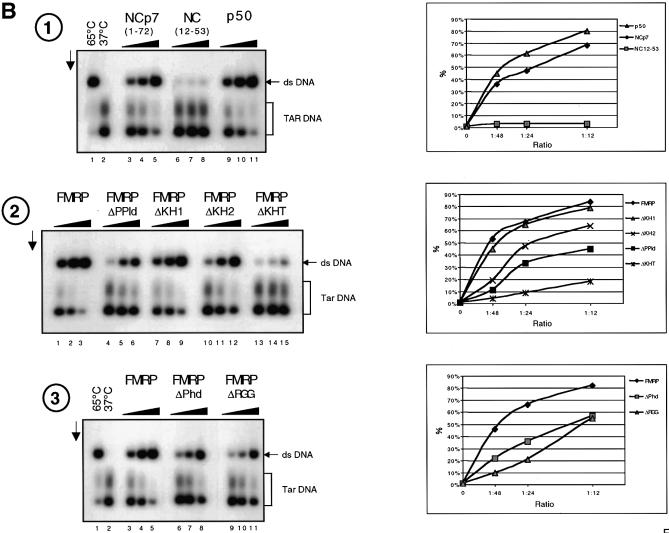
FMRP has nucleic acid annealing activity. (A) Schematic representation of the assay. HIV-1 TAR (+) and (–) DNA sequences are shown. 5′ 32P-labelled TAR(–) DNA is hybridized to TAR(+) upon heating at 65°C or following addition of a nucleic acid chaperone such as HIV-1 NCp7. (B) Annealing assays. Conditions were as described in Materials and Methods. Control hybridization was conducted for 30 min at 65 (lane 1) or 37°C (lane 2). Protein to nucleotide molar ratios were 1:48, 1:24 and 1:12, corresponding to a concentration of 0.35, 0.7 and 1.4 × 10–8 M, respectively. The vertical arrow shows the direction of electrophoresis. 32P-labelled TAR(–) DNA and double-stranded TAR DNA are indicated on the right. (1) NCp7, lanes 3–5; NC(12–53), lanes 6–8; p50, lanes 9–11. (2) FMRP, lanes 1–3; FMRP ΔPPId, lanes 4–6; FMRP ΔKH1, lanes 7–9; FMRP ΔKH2, lanes 10–12; FMRP ΔKHT, lanes 13–15. (3) FMRP, lanes 3–5; FMRP ΔPhd, lanes 6–8; FMRP ΔRGG, lanes 9–11. Curves shown on the right are quantitative assessments of the percentage of double-stranded DNA formed. Note that FMRP is as active as the canonical chaperone HIV-1 NCp7. Only FMRP mutant ΔKH1 was as active as wild-type protein, whereas mutants ΔRGG, ΔKH2, ΔPPId and ΔPhd were ∼2.5–5 times less active and mutant ΔKHT was poorly active (see at molar ratio of 1:48).