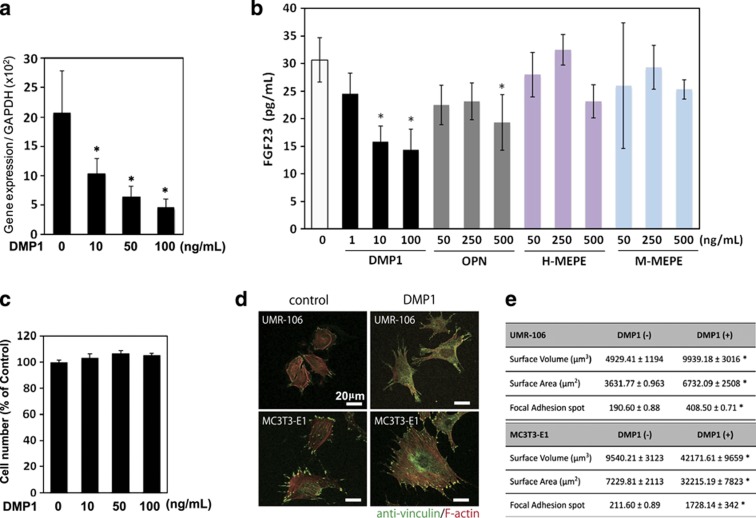
Figure 3.
Downregulation of the fgf23 expression and its protein production by exogenous DMP1. (a, b) UMR-106 cells were cultured with recombinant DMP1 at a concentration of 10, 50 and 100 ng ml−1, and with other RGD-containing proteins, osteopontin, human matrix extracellular phosphoglycoprotein (H-MEPE) and mouse MEPE (M-MEPE) at indicated concentrations. After 24 h, total RNA was extracted from the cells, and the expression levels of fgf23 were analyzed using quantitative real-time PCR. The secreted protein levels of FGF23 in the cell culture supernatant were determined using ELISA. *P<0.05. The data are representative of at least three independent experiments. (c) Effect of the DMP1 treatment on the number of cells was assayed by (3-(4,5,dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) (MTT)-based assay. Effects of exogenous DMP1 on focal adhesion formation and cellular morphology (d, e) UMR106 and MC3T3-E1 cells were treated with or without DMP1 (100 ng ml−1). After 24 h, the cells were fixed and subjected to immunocytochemistry. (d) Anti-vinculin (in green) was used to observe the presence of focal adhesion, and F-actin was stained with tetramethylrhodamine (TRITC)-conjugated phalloidin (in red). (e) The number of focal adhesion points and the cellular volume and surface area were statistically analyzed on the three-dimensional-reconstituted images. *P<0.05. The data are representative of at least three independent experiments. RGD, arginine–glycine–aspartate.