Abstract
Algae biosorption is an ideal wastewater treatment method when coupled with algae growth and biosorption. The adsorption and bioaccumulation of strontium from simulated nuclear wastewater by Scenedesmus spinosus were investigated in this research. One hundred mL of cultured S. spinosus cells with a dry weight of 1.0 mg in simulated nuclear wastewater were used to analyze the effects on S. spinosus cell growth as well as the adsorption and bioaccumulation characters under conditions of 25 ± 1 °C with approximately 3,000 lux illumination. The results showed that S. spinosus had a highly selective biosorption capacity for strontium, with a maximum bioremoval ratio of 76%. The adsorbed strontium ion on cell walls was approximately 90% of the total adsorbed amount; the bioaccumulation in the cytoplasm varied by approximately10%. The adsorption quantity could be described with an equilibrium isotherm. The pseudo-second-order kinetic model suggested that adsorption was the rate-limiting step of the biosorption process. A new bioaccumulation model with three parameters was proposed and could give a good fit with the experiment data. The results suggested that S. spinosus may be a potential biosorbent for the treatment of nuclear wastewater in culture conditions.
Keywords: Scenedesmus spinosus, biosorption, adsorption, bioaccumulation, strontium, kinetics model
1. Introduction
The biosorption of heavy metals by living or growing biomasses involves two processes: an initial rapid, passive adsorption followed by a much slower active bioaccumulation process. The former is a metabolism-independent process, while the latter is metabolism-dependent [1,2]. The majority of studies on the bioaccumulation of heavy metal ions by microorganisms only consider the final concentration of metal ions, and only a few studies provide quantitative kinetic descriptions of the process and investigate its mechanisms [3]. Thus, the investigation of adsorption and bioaccumulation processes and their mechanisms can facilitate application of these techniques in wastewater treatment or other associated fields.
Krejci et al. [4] have found that a common freshwater alga (Closterium moniliferum) showed the notable ability to bioremove strontium from water and proposed a “sulfate trap” model by preferential precipitation of (Ba, Sr)SO4 due to its lower solubility compared to SrSO4 and CaSO4. It is believed that this discovery can help scientists design suitable methods to bioremove radioactive strontium from existing nuclear wastewater streams as many algae have immense metal ion biosorption capabilities [2]. The metal biosorption capacity of an algal biomass is comparable, or sometimes higher, than that of chemical sorbents [2,5]. In addition to the higher biosorbing capacity, algae are autotrophs, which can grow in wastewater with little or no nutritional supplements [2]. The coupling of the algal growth and biosorption of harmful metal ions from wastewater under culture conditions make algal biosorption an ideal metal ion scavenging method [6,7]. Scenedesmus spinosus is a common type green alga that typically consists of 4–8 cells aligned in a flat plate with single short spine arising at each pole of terminal cells and one spine at the middle of terminal cell [8]. S. spinosus is abundant in many natural water environments and shows good tolerance to heavy metal pollution [9,10,11]. S. quadricauda is commonly used for a variety of studies in different fields of science [5], but there are few publications regarding S. spinosus.
There are some published studies that investigated the capacity of Scenedesmus to biosorb heavy metal ions from solutions. S. quadricauda showed a highly efficient sequestration capacity of Ni+ and Cu2+ [2,12]. Terry and Stone [10] have shown that while both living and nonliving S. abundans removed cadmium and copper from water, living algae significantly outperformed nonliving algae. Omar [13] has found that the maximum specific adsorptive capacity of zinc ions obtained from the Langmuir adsorption isotherms was higher for S. obliquus (6.67) and lower for S. quadricauda (5.03) and that S. obliquus was more tolerant of zinc phytotoxicity than S. quadricauda. A Wuhan isolate of Scenedesmus could tolerate a mixture of 30 mg∙L−1 Ni2+ and 30 mg∙L−1 Zn2+ in wastewater and could remove most of the Ni2+ and Zn2+ from the simulated wastewater in 5 min [14]. S. quadricauda had a high biosorption capacity for Ni2+, Cu2+ and Cu+, while the biosorption capacity was lowest for Mo6+ of the six metals (Cu2+, Cu+, Mo6+, Mn2+, V5+, Ni2+), both individually and when in combined solutions of these metals [15]. The immobilized S. quadricauda showed a good adsorption capacity for Cu2+, Zn2+ and Ni2+ ions and was particularly selective for Cu2+ ions [5,16].
Biosorption with microorganisms under culture conditions may facilitate the development of continuous industrial treatment of contaminated wastewater [17]; however, as described above, there are few publications concerned with the treatment of liquid wastes with low or intermediate levels of radioactivity with S. spinosus under culture conditions.
The present research has investigated the bioremoval efficiency of strontium ions from the simulated nuclear wastewater by S. spinosus under culture conditions and analyzed the adsorption and bioaccumulation characteristics, processes, kinetics and models.
2. Experimental Section
2.1. Scenedesmus spinosus and Culture Method
The Scenedesmus spinosus was isolated from the wastewater samples which were obtained from the Tianfu Changcheng pool, Chengdu, China. The culture medium was a modified Chu No. 10 nutrient solution: Ca(NO3)2 0.04 g; K2HPO4 0.01 g; MgSO4·7H2O 0.025 g; Na2CO3, 0.02 g; NaSiO3 0.025 g; Soil extract 20 mL; F/2Vitamin stock solution, 1 mL; water, 1,000 mL; pH 6.5–7.0. The culture was incubated statically at 25 ± 1 °C. Incubation was performed under a continuous light intensity of approximately 3,000 lux, which was produced by two 40-W white fluorescent lamps placed above the Erlenmeyer flasks. During the culturing period, the Erlenmeyer flasks containing the cultures were shaken twice a day, the culture medium was replaced every 3 days, and the pH was allowed to drift freely. Culturing was performed over a period of approximately 7 days, and the cultured S. spinosus was then prepared for biosorption.
2.2. Reagents
All chemical reagents used were of analytical grade. The strontium stock solution (500 mg∙L−1) was prepared by dissolving 1.2575 g of strontium nitrate (Sr(NO3)2) in 1,000 mL of distilled deionized water. A simple solution containing only strontium ions was prepared by diluting the stock solution to a preset concentration.
The components of the simulated low- and intermediate-level radioactive liquid waste (simplified as simulated wastewater) are listed in Table 1 [18]. In this research, the strontium concentration was reduced below the reference data because when the strontium concentration is larger than 200 mg∙L−1, other constituents in the medium, such as sulfate, carbonate and phosphate ions, may react with strontium ions to form sediments [19]. The strontium ion concentration was varied from 0 to 100 mg∙L−1 to analyze the effects of coexisting ions on the biosorption of strontium.
Table 1.
The simulated low and intermediate level radioactive liquid waste components.
| Elements | Nitrates | Contents/g∙L−1 |
|---|---|---|
| Na | NaNO3 | 31.560 |
| Al | Al(NO3)3 | 13.670 |
| Fe | Fe(NO3)3 | 7.460 |
| Cr | Cr(NO3)2 | 0.960 |
| Ni | Ni(NO3)2 | 2.670 |
| K | KNO3 | 0.620 |
| Ba | Ba(NO3)2 | 0.014 |
| Sr | Sr(NO3)2 | 0–0.1 |
| Cs | CsNO3 | 0.175 |
2.3. Biosorption of Strontium Ions by S. spinosus under Batch Culture Conditions
The simple solutions or simulated wastewater solutions (20 mL) were mixed with suspensions of growing S. spinosus (40 mL, cell dried weight: 1.0 mg∙L−1) and the culture medium (40 mL) prepared as described above. Then, the mixture continued to culture. After a preset biosorption time (11 time gradients from 1 to 144 h for simple solutions and six time gradients from 24 to 144 h for simulated wastewater solutions), the cell suspensions were centrifuged (22 °C, 15 min, 4,000 rpm). The supernatants were collected for analysis of the residual metal ion concentrations, and the sediment was collected for analysis of the adsorption and bioaccumulation of strontium ions by S. spinosus cells.
2.4. The Adsorption and Bioaccumulation of Strontium on S. spinosus Cells Analysis with Wet Ashing Method
A brief description of the wet ashing method follows [20]. First, the S. spinosus cell sediments collected after the biosorption phase were washed with distilled, de-ionized water to remove all of the unbound strontium ions from the S. spinosus cells. After washing, each sediment sample was suspended in 2 mL of 0.01 mol∙L−1 EDTA and agitated for 5–10 min, and then the mixtures were centrifuged for 20 min at 10,000 rpm. The supernatants were then collected. The centrifugation and supernatant collection process was repeated twice, and the three supernatant samples collected were then combined to measure the strontium ion concentration adsorbed by the S. spinosus cell walls. The cell sediments collected after washing with EDTA were digested with 2 mL of nitrification solutions (HClO:HNO3 = 1:4) for 48 h. After digestion, the mixtures were centrifuged for 20 min at 10,000 rpm; the supernatants were then collected to measure the concentration of strontium ions intracellularly bioaccumulated by the S. spinosus cells.
2.5. Metal Ions Concentration Measurement and Data Analysis
Timed sample supernatants of 10 mL were used to assay residual metal ion concentrations with an atomic absorption spectrophotometer (PE AA700, Shelton, CT, USA) at the Analytical and Testing Center, Southwest University of Science and Technology. The bioremoval ratio (R) was calculated using the following formula:
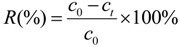 |
(1) |
The biosorption quantity of strontium (q) was calculated with the following equation:
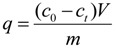 |
(2) |
where q (mg∙g−1) is the amount of strontium adsorbed per gram of dry weight, c0 (mg∙L−1) is the initial concentration of strontium, ct (mg∙L−1) is the concentration of the strontium in solution at the preset culture time, V (L) is the volume of solution, and m (g) is the dry mass of S. spinosus.
The quantity of strontium ions adsorbed in the S. spinosus cell walls was calculated using the following equation:
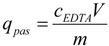 |
(3) |
where qpas (mg∙g−1) is the adsorption quantity of strontium ion in the S. spinosus cell walls, cEDTA (mg∙L−1) is the strontium concentration in the EDTA washing solution, V (L) is the volume of the EDTA washing solution, and m (g) is the dry mass of S. spinosus.
The quantity of strontium ions bioaccumulated in the S. spinosus cytoplasm was calculated as follows:
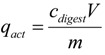 |
(4) |
where qact (mg∙g−1) is the quantity of strontium ions bioaccumulated in the S. spinosus cytoplasm, cdigest (mg∙L−1) is the strontium concentration in the digest solution, V (L) is the volume of the digest solution, and m (g) is the dry mass of S. spinosus.
When performing calculations for the bioaccumulation model, ct, qpas and qact were written as cMe, cpas and cact, which represent the free strontium ions in solution, the strontium ion concentrations on the S. spinosus cell walls and the strontium ion concentrations in the S. spinosus cytoplasm, respectively.
2.6. Modeling
The parameters of the nonlinear isotherm models and bioaccumulation model were determined by linear programming; the arithmetic used is as follows:
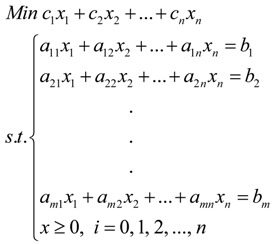 |
(5) |
3. Results and Discussion
3.1. The Effects of Strontium Ions in Simulated Wastewater on S. spinosus Cell Growth
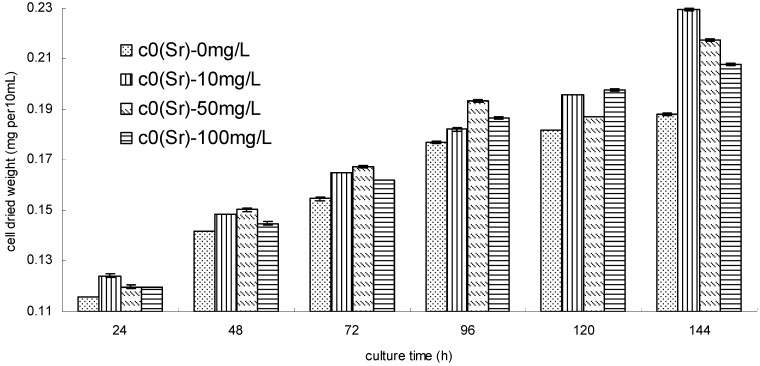
Observation under a microscope showed that the S. spinosus cells that were separated from the wastewater grew well in the simulated nuclear wastewater. The results in Figure 1 show that the low concentration of strontium ions in the simulated wastewater could stimulate the growth of S. spinosus cells. The cell dried weight approximately doubled in the 144-h culture stage. A similar result was obtained for Dicratetia inornata and Platymonas subcordiformis, which can tolerate strontium ion concentrations greater than 1.44 mmol∙L−1 [21,22]. The D. inornata growth was severely inhibited when the initial strontium concentration reached 5.76 mmol·L−1 (approximately 500 mg∙L−1) [21]. The S. spinosus growth stimulation by the simulated wastewater may be due to the high concentrations of ferric, sodium and other ions. Liu et al. [23] have shown that a ferric ion concentration of 3,000 nmol∙L−1 could also enhance the growth of S. quadricauda cells.
Figure 1.
The effect of simulated wastewater on S. spinosus growth during the biosorption phase (S. spinosus cell dried weight per 10 mL of culture medium).
3.2. The Bioremoval Ratio of Strontium Ions from Simulated Wastewater by S. spinosus under Batch Culture Conditions
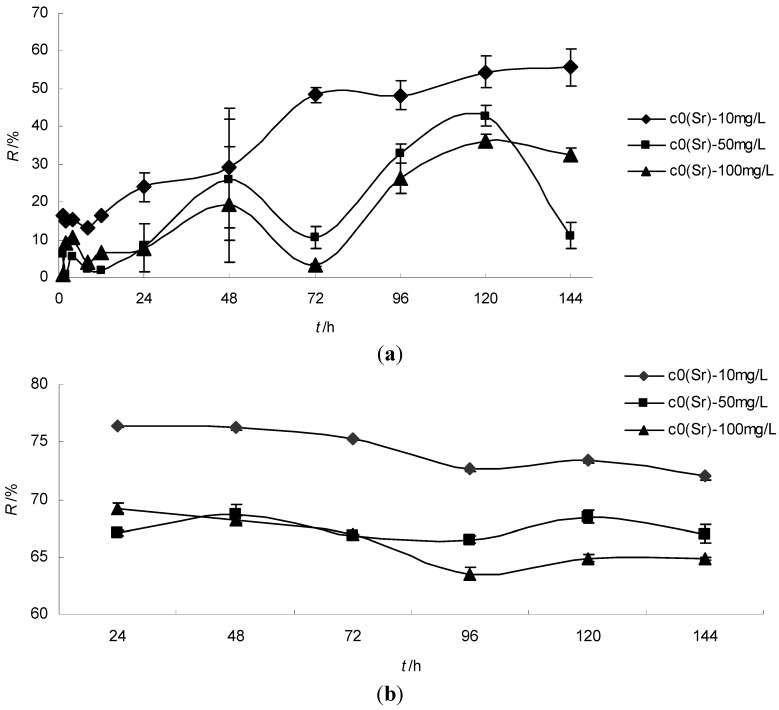
To investigate the biosorption of strontium ions from the simulated nuclear wastewater, biosorption of strontium ions from a simple solution containing a single strontium ion was first carried out (Figure 2a). The results showed that the bioremoval ratio of strontium ions varied with the culture time. The bioremoval ratio of strontium ions increased with culture time when the initial strontium ion concentration was 10 mg∙L−1; a maximum removal ratio of approximately 60% was obtained. The strontium ion removal behavior was similar for initial strontium ion concentrations of 50 mg∙L−1 and 100 mg∙L−1. The results suggested that the S. spinosus had a low efficiency for bioremoval of strontium ions from a simple solution.
Figure 2.
(a) The bioremoval ratio of strontium ions from a simple solution containing single strontiumions by S. spinosus under batch culture conditions; (b) The bioremoval ratio of strontium ions from simulated wastewater by S. spinosus under batch culture conditions.
Because the bioremoval ratio was very low during the first 24 h for strontium ion bioremoval from the simple solution, a process for the biosorption of strontium ions from simulated wastewater was designed from 24 to 144 h.
The results showed that the bioremoval of strontium ions from simulated wastewater was different compared to that from a simple solution. Figure 2b shows that the removal of strontium ions was a quick process; the biosorption reached equilibrium in 24 h, and the removal ratio was above 60%, with a maximum removal ratio of approximately 76%. For an initial concentration of 10 mg∙L−1, the removal ratio increased with the culture time in simple solution conditions, while it decreased in simulated wastewater. For initial concentrations of 50 and 100 mg∙L−1, a periodic oscillation of the removal ratio, with a period of approximately 72 h, was observed. A similar trend was observed for the biosorption of Cd2+ by Chlorella pyrenoidosa [24].
3.3. The Co-Existing Cations Effects on Biosorption of Strontium Ions in Simulated Wastewater
Factors that affect biosorption include pH, ion mixing, time, temperature, pretreatment and biomass quantity. Co-existing metal ions mainly affect the biosorption of strontium ions from wastewater [25]. In this research, the co-existing cations were treated as a whole; the strontium ion concentration was varied to analyze the co-existing cations’ effect on strontium ion adsorption and bioaccumulation.
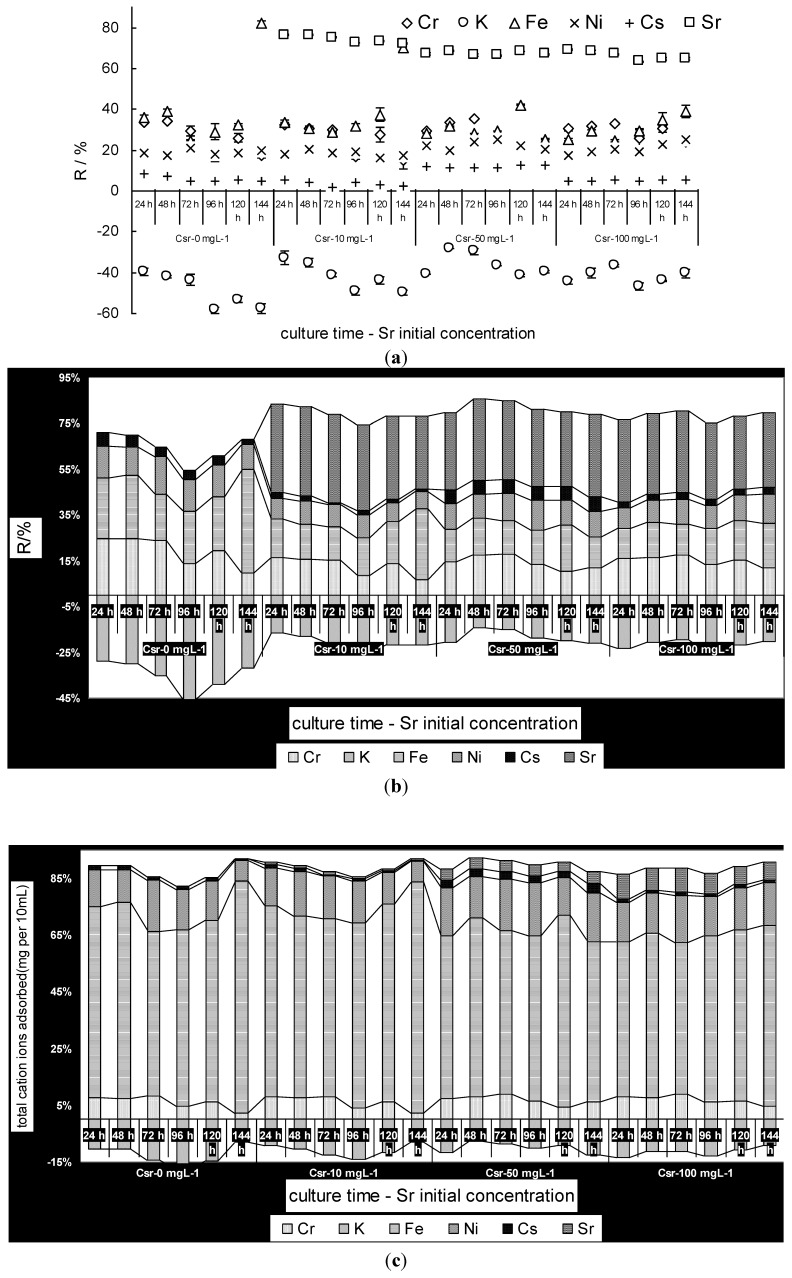
The various co-existing cations investigated in simulated wastewater showed different effects on strontium ion biosorption in this study. The S. spinosus cells displayed a great bioremoval ratio for strontium, ferric and chromium ions; a negative bioremoval ratio for potassium ions; and a small bioremoval ratio for nickel and cesium ions (Figure 3a). During the first 72-h biosorption course, the strontium ions caused the bioremoval ratio of the other cations to decrease. Additionally, the bioremoval ratios varied with the initial strontium ion concentration. During the next 72-h biosorption course, the strontium ions increased the bioremoval ratio of the other cations. A periodic oscillation of the bioremoval ratios was seen for other cations, similar to that of the strontium ions. The total ion quantity adsorbed by S. spinosus when strontium ions were added to the simulated wastewater was more stable than the quantity with no strontium ions in the simulated wastewater during the 144-h bisorption course. The total ion quantity adsorbed increased greatly in the 144-h biosorption course due to the large increase in the biosorption of ferric ions. The packing arrangement figure (Figure 3b,c) of adsorbed ions quantity per 10 mL showed that the ferric ions were the largest cations, followed by nickel and chromium ions. S. spinosus cells adsorbed few cesium ions. The strontium ion concentration was the lowest in the simulated wastewater (below 0.1 g∙L−1). However, the S. spinosus cells had a high bioremoval ratio for strontium ions, and a relatively high adsorption quantity. These results suggested that the S. spinosus cells showed a strong selective biosorption capacity for strontium ions. The other cations’ bisorption was dependent on the ion concentration. The negative removal ratio of potassium ions suggests that biosorption of other metal ions caused an ion-exchange with potassium ions. The small biosorption quantity for cesium ions may be due to the small bisorption capacity for monovalent cations. The results showed that the total biosorbed ion quantity increased with the initial strontium ion concentration, and the quantity in the different culture stages was almost at the same level. This suggested that the biosorption capacity of S. spinosus of metal ions was dependent on the ions’ concentrations in the solution.
Figure 3.
(a) The packing arrangement figure of the co-existing cation bioremoval ratio (R); (b) The packing arrangement figure of adsorbed cation quantity per 10 mL (q); (c) The total adsorbed cation quantity per 10 mL (q).
The grey relational grade analysis showed that the order for different co-existing metal ion effects on strontium ion biosorption was Fe3+ > Cr3+ > Ni2+ > K+ > Cs+.
The biosorption of metal ions by algae is a larger concern for single, heavy metal ions such as Cd2+, Ni2+, Cr3+, Cu2+ [24,26,27]. These studies also agree with the present research results that the amount of metal ions absorbed by the alga increased with higher initial metal ion concentrations [24,26,27]. The sorption capacity of the chlorella for Cd2+ was much higher than that for Cu2+ and Cd2+ [28]. Increasing the Cu2+ concentration could increase the biosorption of Cr4+ by Rhizopus nigricans [29]. However, prior research has shown the poor performance of metal ions in simulated wastewater. In the present research, the performance of multi-metal ions in simulated wastewater was analyzed. The results showed that the S. spinosus displayed a great bioremoval ratio for strontium ions compared to other co-existing cations’ bioremoval ratio. The reason may be that metal ions of Fe3+ in the simulated wastewater could promote the S. spinosus growth [23], thereby increasing the strontium scavenging ratio compared to simple solutions. The toxicity of Ni2+, Cr2+ and Cs+ metal ions [26] not only showed a low inhibiting effect on the growth and morphology of S. quadricauda but also caused a low scavenging ratio by S. quadricauda cells. That is, that single poisonous metal ion was no more toxic when combined in the same system. This may explain why many algae can naturally grow well in polluted waters. This also proved the opinion that actinide toxicity is primarily chemical (not radiological) and that radiation resistance does not ensure radionuclide tolerance [30]. Because the radioactivity of low- and intermediate-level radioactive liquid waste is low, algae can endure the chemical toxicity and grow well in these media; this fact facilitates the application of algae for radioactive nuclides wastewater treatment.
3.4. The Adsorption and Bioaccumulation of Strontium Ions by S. spinosus Cells
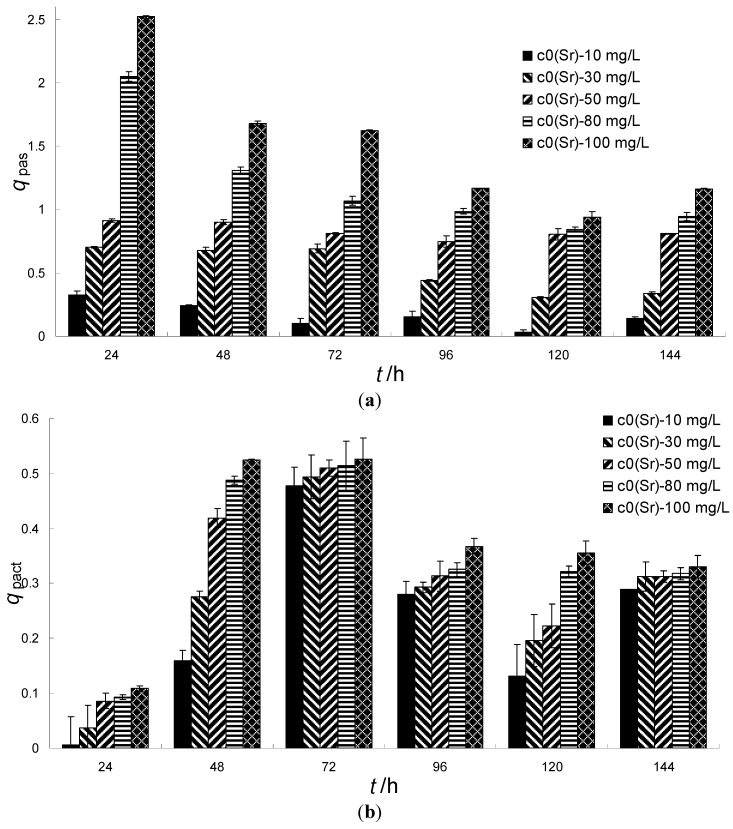
The cell walls of algae have the capacity to bind metal ions in negatively charged sites [31,32]. However, the algae also have the ability to accumulate the metal ions through metabolism under culture conditions. Figure 4a showed that the adsorption of strontium ions by S. spinosus was a very rapid process when the S. spinosus was cultured in simulated wastewater. The qpas reached its peak in the first 24 h, which suggests that the adsorption process is a physical reaction that occurs early in the adsorption course and the cell walls were the first sites for adsorbing. When the biosorption process continued, qpas decreased with culture time. The qpas value also increased with the initial strontium ion concentration at different culture stages. The adsorption process can be divided into two stages: during the first 72-h stage, the qpas was high; during the next 72-h stage, the qpas value was low. This change may be explained as follows: (1) The adsorbed strontium ions were transported into the cytoplasm through metabolism; (2) The cell weight increased. For example, the cell weight increased from 0.09 g per 10 mL at 24 h to 0.24 g per 10 mL at 144 h; (3) The cell may have acquired a resistance to strontium ions that led to desorption of adsorbed strontium ions.
Figure 4.
(a) The adsorption of strontium ions by S. spinosus cells from simulated wastewater (qpas-t); (b) The bioaccumulation of strontium ions by S. spinosus cells from simulated wastewater (qact-t).
The bioaccumulation qact was very small during the first 24 h and increased during the next 48 h (Figure 4b). In the following 72 h, qact decreased noticeably. In general, qact increased with the initial strontium ion concentration, but with longer residence times, this correlation diminished. For example, qact reached nearly the same value after 72 h and 144 h. This suggested that the bioaccumulation of metal ions did not depend on the initial metal ion concentration, and the bioaccumulation qact may mainly depend on the metabolism of the cell and the cell’s tolerance of the metal ions. In the first 72 h, the main reaction was bioaccumulation through metabolism, so qact increased rapidly. The decrease in qact may be due to the decrease in qpas described above. Thus, these results suggested that the biosorption of metal ions may be a time-dependent process under culture conditions, and an optimal biosorption time existed that varied with the biosorbent and metal ion types, which is different from biosorption by the traditional method. The similar results were obtained from biosorption of cadmium by C. pyrennoidosa. The uptake of cadmium by C. pyrennoidosa occurred in at least two phases: fast (8 min) and slow (24 h). The fast phase consisted of ion-exchange uptake onto the cell wall, while the slow phase involved transport across the cell membrane [24].
The different initial strontium ion concentrations could yield nearly the same level of qact, suggesting that the cell had a saturated bioaccumulation quantity that did not depend on qpas. The decrease in qact may be due to the cell initially developing accommodation sites for the ions and then opening an ion channel to transport the strontium ions out of the cytoplasm.
The data also showed that the adsorbed strontium ions on the cell wall occupied over 90% of the available ions early in the biosorption process and then decreased to approximately 70% later in the biosorption stage. Latha et al. [33] showed that the Neurospora crassa surface accommodates approximately 90% of cobalt ions. Candida utilis cell walls accumulated 50% of the copper ions and it was suggested that the cell wall was the main site for heavy metal accumulation [34]. The La3+, Eu3+, Yb3+ lanthanide ions that accumulated on Pseudomonas aeruginosa cell walls accounted for approximately 90% of the total ion uptake [35]. However, it was interesting to find that the intracellular strontium ratio for a low initial strontium concentration was larger than that for a high initial strontium concentration. The reason for this result may be that the low concentration strontium is less toxic to S. spinosus cells, giving the S. spinosus cells a larger bioaccumulation capacity.
3.5. Equilibrium Isotherm
The capacity of a biosorbent can be described by certain constants of the equilibrium sorption isotherm, the values of which express the surface properties and affinity of the biosorbent [36]. To analyze the validity of the adsorption data, the most common adsorption isotherm models (Langmuir, Freundlich, etc.) were applied, as discussed below. The common biosorption equilibrium isotherm was modeled with qe data calculated according to Equation (2). In this study, we have assayed the cell wall adsorption quantity qpas. Thus, we fit the equilibrium isotherm with qpas.
The Langmuir equation and linear expression were respectively describled as follows [37]:
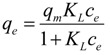 |
(6) |
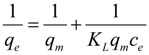 |
(7) |
where qe is the amount adsorbed (mg∙g−1), ce is the equilibrium concentration of the adsorbate (mg∙L−1), qm is the Langmuir constants related to the maximum monolayer adsorption capacity (mg∙g−1), and KL is the constant related to the free energy or net enthalpy of adsorption.
The Freundlich isotherm equation and logarithmic form were respectively describled as follows [38]:
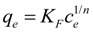 |
(8) |
| ln qe = ln KF + (1 / n)ln ce | (9) |
where KF (L∙g−1) and n indicate the affinity of the adsorbate to the biomass. Therefore, the values of KF and 1/n were calculated from the plots of ln qe versus ln ce.
The three-parameter isotherm model Koble-Corrigan equation was describled as follows [39]:
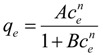 |
(10) |
where A (L·g−1), B and n were the Koble-Corrigan isotherm parameters.
Besides the r2, Chi-square test was adopted to assess the suitability of selected isotherm model. The Chi-square test equation was described as follows [40]:
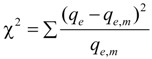 |
(11) |
where qe,m (mg·g−1) is the calculated adsorbed amount based on the model parameters.
The Langmuir biosorption isotherm was more suitable for the estimation of the monomolecular maximum adsorption capacity. The monomolecular adsorption capacity qm (Table 2) showed that the S. spinosus cell walls had nearly the same adsorption capacity in different culture stages. The dimensionless equilibrium parameter (RL) was used to estimate the affinity between the sorbate and sorbent [41]. The RL expression is:
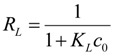 |
(12) |
where KL is the Langmuir constant and c0 is the initial strontium ion concentration. The different value of RL indicated the different type of Langmuir isotherm: irreversible (RL = 0), linear (RL = 1), unfavorable (RL > 1), or favorable (0 < RL < 1) [41]. The RL value was from 0.28952 for c0 100 mg∙L−1 at 24 h to 0.85049 for c0 10∙mg·L−1 at 48 h. These results indicated that the adsorption of the strontium ion onto S. spinosus cells is favorable [41]. The parameters in Table 2 regressed with linear or nonlinear method suggested that the linear isotherm regression and nonlinear isotherm regression both completely fitted the experimental data based on r2 and χ2.
Table 2.
The equilibrium isotherm model parameters.
| Model | Time (h) | Linear Isotherm Regression | Nonlinear Isotherm Regression | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Parameters | r2 | χ2 | Parameters | r2 | χ2 | |||||
| qm | KL | qm | KL | |||||||
| Langmuir | 24 | 2.35627 | 0.06618 | 0.9531 | 0.8864 | 7.65490 | 0.01374 | 0.9262 | 0.1902 | |
| 48 | 2.34082 | 0.04904 | 0.9966 | 0.0594 | 4.52553 | 0.01758 | 0.9780 | 0.0498 | ||
| 96 | 2.62950 | 0.02242 | 0.9998 | 0.0019 | 2.44126 | 0.02518 | 0.9985 | 0.0018 | ||
| 144 | 2.50188 | 0.02127 | 0.9868 | 0.0597 | 2.89086 | 0.01908 | 0.9668 | 0.0413 | ||
| Freundlich | n | KF | n | KF | ||||||
| 24 | 1.29702 | 0.43837 | 0.9311 | 7.6716 | 0.85574 | 0.04543 | 0.9548 | 0.4544 | ||
| 48 | 1.39179 | 0.41849 | 0.9924 | 6.8841 | 1.35220 | 0.12624 | 0.9885 | 0.0160 | ||
| 96 | 1.25565 | 0.31886 | 0.9922 | 9.4048 | 1.43487 | 0.09741 | 0.9910 | 0.0156 | ||
| 144 | 1.16918 | 0.29145 | 0.9748 | 10.8071 | 1.32003 | 0.07969 | 0.9581 | 0.0556 | ||
| Koble-Corrigan | A | B | n | |||||||
| 24 | 0.03265 | −0.89004 | 0.02991 | 0.9721 | 0.0744 | |||||
| 48 | 0.16437 | −0.10717 | 0.45695 | 0.9928 | 0.0171 | |||||
| 96 | 0.05230 | 0.02496 | 1.08766 | 0.9988 | 0.0015 | |||||
| 144 | 0.02130 | 0.01346 | 1.47458 | 0.9719 | 0.0568 | |||||
The Freundlich biosorption isotherm was based on the biosorption of a heterogeneous surface. The values of KF were found to be decreasing with culture time. The nonlinearity degree between metal ion concentration and adsorption can be determined by n value: linear (n = 1), poor adsorption capacity (n < 1), good adsorption capacity (n > 1) [41]. The n value showed in Table 2 was 1.16918–1.39179. The linear isotherm regression values of n > 1 indicated positive binding and a heterogeneous nature of adsorption of the S. spinosus cell walls [41]. The r2 results (Table 2) showed that the linear isotherm regression and linear isotherm regression both fitted the experimental data. But the χ2 results suggested that the nonlinear isotherm regression was more suitable for accurately describing the Freundlich isotherm relationship than the linear isotherm regression.
Compared to two-parameter isotherm models, the three-parameter isotherm model Koble-Corrigan was the best fitted isotherm model based on r2 and χ2 parameters. These results suggested that the strontium ion biosorption onto S. spinosus cells was a complex process following a combined isotherm models [42].
The cell wall is the main site for biosorption of metal ions, but the cell may transport some metal ions into the cell’s interior under culture conditions by metabolism. Thus, the equilibrium isotherm model should be described using the higher resolution data of the cell wall-adsorbed ion quantity instead of the total biosorption quantity [1]. For example, Ergene et al. [43] elucidated the equilibrium isotherm model of immobilized active and inactive S. quadricauda for dye removal using the qe, which was calculated based on the changes in the initial and equilibrium ion concentrations. The qpas data were used to elucidate the equilibrium isotherm model in the present research. The results suggested that the model could be used to evaluate the metal ion adsorption on the cell surfaces. The results also indicated that the adsorption of the metal ions by S. spinosus cell walls followed the isotherm laws. It was showed that the nonlinear isotherm regression method is a better way to obtain the isotherm parameters.
3.6. Kinetic Models for Biosorption
To determine the metal ion biosorption kinetics, the Ritchie pseudo-second-order kinetic model was used; the equation is shown below [44,45,46]:
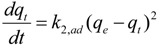 |
(13) |
Integrating and rearranging with the initial condition qt = 0 at t = 0, the following equation is obtained:
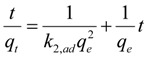 |
(14) |
where dqt/dt is the initial biosorption rate (mg∙g−1∙min−1) and is defined as t→0 by h = k2,adqe2, and k2,ad is the pseudo-second-order biosorption rate constant (g∙mg−1∙min−1). The value qe is determined from the slope of t/qt versus t and h is determined from the intercept [44].
The Lagergren pseudo-first-order and Ritchie pseudo-second-order kinetic models were used for estimation of the biosorption kinetics [47,48]. The data for the present research did not fit the Lagergren pseudo-first-order model, so the data are not included in Table 3. To analyze the biosorption kinetics, qe based on the scavenging ratio data, qpas based on the adsorption data and qpas+act based on the adsorption adding bioaccumulation data were used to fit the model. The rate parameters for the biosorption of the strontium ions by the S. spinosus cells are presented in Table 3. Analysis of r2 showed that the data based on the scavenging ratio was more suitable for the pseudo-second-order model than the data based on adsorption and adsorption with bioaccumulation. This result suggested that the model was more suitable for describing the total ion changes in the adsorption system. This result indicated that adsorption may be the rate-limiting step of the biosorption process [43].
Table 3.
The kinetic model parameters.
| Calculation Model | The Kinetics Model Parameters | ||||
|---|---|---|---|---|---|
| Based on scavenging ratio(R) | c0(sr) | qe | k2 | h | r2 |
| 10 mg∙L−1 | 0.29345 | −0.17085 | −0.01471 | 0.9740 | |
| 50 mg∙L−1 | 1.45264 | −0.04289 | −0.09050 | 0.9831 | |
| 100 mg∙L−1 | 2.80110 | −0.02057 | −0.16139 | 0.9932 | |
| Based on qpas+qact | c0(sr) | qe | k2 | h | r2 |
| 10 mg∙L−1 | 0.25839 | −0.26045 | −0.01739 | 0.4950 | |
| 50 mg∙L−1 | 1.04998 | −0.18523 | −0.20420 | 0.9759 | |
| 100 mg∙L−1 | 1.23870 | −0.04302 | −0.06600 | 0.9620 | |
| Based on qpas | c0(sr) | qe | k2 | h | r2 |
| 10 mg∙L−1 | 0.05534 | −0.69997 | −0.00214 | 0.3631 | |
| 50 mg∙L−1 | 0.77286 | −0.35743 | −0.21350 | 0.9937 | |
| 100 mg∙L−1 | 0.92618 | −0.05466 | −0.04688 | 0.9465 | |
3.7. Models for Bioaccumulation
The pseudo-second-order model was well suited for description of the total ion concentration change kinetics, and the equilibrium models were suitable for the description of the cell wall adsorption quantity. There are only a few models describing bioaccumulation in the literature [3]. A two-step bioaccumulation process was proposed that afforded a quick binding of metal ions to the cell walls, followed by a slower transport process through the cell membrane [3].
Based on the following physical model, Chojnacka and Wojciechowski [3] proposed a mathematical kinetics model:
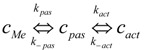 |
(15) |
On the basis of microscopic observations, it was observed that during microbial growth, the ratio of cellular mass to their surface area is constant. The equation of mass balance and kinetic equations describing concentrations of all forms of strontium ions were proposed:
| cMe (t) + cpas (t) + cact (t) = cMe (t = 0) | (16) |
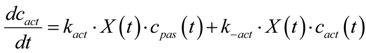 |
(17) |
However, when this model was used to analyze the experimental data, a good mathematical model could not be obtained. From Equation (17), the following bioaccumulation kinetics model was proposed based on the biosorption and bioaccumulation mechanisms in the present study:
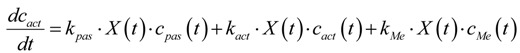 |
(18) |
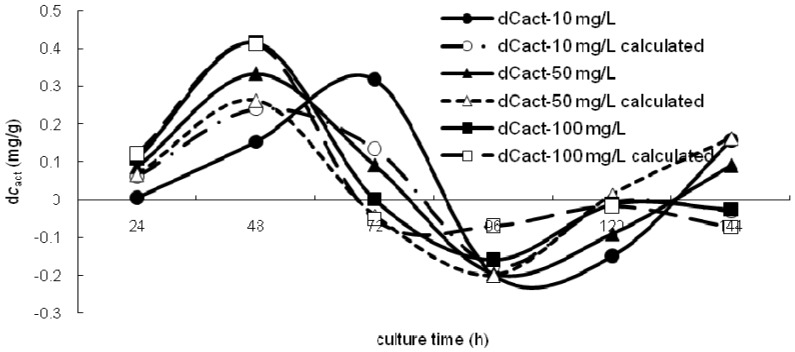
In this model, three parameters kpas, kact and kMe were used to describe the effects of the three concentrations, cpas, cact and cMe, on the bioaccumulation of the S. spinosus cells. An additional cMe factor, compared to Chojnacka’s model, was added to describe the effects of the free strontium ion concentration in the solution on the bioaccumulation of the S. spinosus cells. Then, a dynamic programming approach was used to elaborate the experimental data. Figure 5 show that the modeled data could fit the experimental data well. The parameters that were used are listed in Table 4.
Figure 5.
The dcact and dcact with calculated data for the bioaccumulation model.
Table 4.
The bioaccumulation model parameters.
| Parameter | kpas | kact | kMe | r2 | |
|---|---|---|---|---|---|
| c0(Sr) | 10 mg∙L−1 | 5.5784 | −3.7626 | 0.0616 | 0.4936 |
| 30 mg∙L−1 | 3.7022 | −7.1359 | 0.0243 | 0.7292 | |
| 50 mg∙L−1 | 2.8420 | −7.2902 | 0.0135 | 0.7628 | |
| 80 mg∙L−1 | 1.7891 | −5.9332 | 0.0109 | 0.9570 | |
| 100 mg∙L−1 | 1.4800 | −6.1437 | 0.0122 | 0.9316 | |
The results showed that the concentration of strontium ions on the cell walls (cpas) was positively related to the strontium ion transport into the cytoplasm. Conversely, the concentration of strontium ions in the cytoplasm (cact) was negatively related to the strontium ions’ entrance into the cytoplasm. The reason for these relations may be that strontium ions on the cell walls cause a concentration stress that increases transport, but a high concentration of strontium ions in the cytoplasm may harm the cell, causing the cell to attempt to transport the ions out of the cytoplasm. Free strontium ions in the solution may exert a certain effect on bioaccumulation. Thus, this model can be summarized in the following manner:
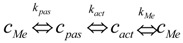 |
(19) |
In this model, the free strontium ions may directly enter or be transported out of the cytoplasm into the culture medium by cell transmembrane transport processes [3,35]. This is different from the mathematical kinetics model proposed by Chojnacka and Wojciechowski [3] (Equation (17)). Their model shows that the free ions affected the bioaccumulation through the cpas, meaning that metal ions first bonded to cell wall and were then transported into the cytoplasm. There are many means of cell transmembrane transport. For example, P1B-type ATPases can transport a number of heavy metals ions, such as Cu+, Cu2+, Ag+, Zn2+, Cd2+, Pb2+ and Co2+, across biological membranes; these transporters are found in archaea, bacteria and eukaryote cells required for maintaining metal ions homeostasis [49,50]. However, these studies did not indicate the location of efflux ions on the cell wall or into the surrounding environment. Thus, future research needs to accurately measure the relationship between cpas, cact, cMe and the cell transmembrane transport system activities.
4. Conclusions
Scenedesmus spinosus showed a strong capability for selective adsorption and bioaccumulation of strontium ions from simulated wastewater under culture conditions. The results showed that S. spinosus had a highly selective biosorption capacity for strontium with a maximum bioremoval ratio of 76%. The grey relational grade analysis showed that the order for different co-existing metal ion effects on strontium ion biosorption was Fe3+ > Cr3+ > Ni2+ > K+ > Cs+. The adsorbed strontium ion on cell walls was approximately 90% of the total adsorbed amount; the bioaccumulation in the cytoplasm varied by approximately 10%. The isotherm model Slips best fitted the experimental data and indicated that the adsorption of strontium ions onto S. spinosus cells was a complex process. The pseudo-second-order kinetic model suggested that adsorption was the rate-limiting step of the biosorption process. A new bioaccumulation model with three parameters qpas, qact and cMe was proposed and can yield a good fit with the experiment data. These results suggest that S. spinosus may be a potential biosorbent for treatment of nuclear wastewater under culture conditions.
Acknowledgments
The authors thank the National Nature Science Foundation of China (Grant No.: 41272371, 41102213), the National Basic Research Program of China (973 Program: 2014CB846003) and the China Academy of Engineering Physics for a NSAF grant (Grant No.: 11176028); thank Yan Luo, Xia Chen from Life Science and Engineering College, Southwest University of Science and Technology for research works.
Author Contributions
All co-authors contributed to the planning of the project and reviewed the manuscript. The initial manuscript draft was written by Mingxue Liu and further improved by co-authors of Faqin Dong, Wu Kang, Shiyong Sun, Hongfu Wei, Wei Zhang, Xiaoqin Nie, Yuting Guo, Ting Huang and Yuanyuan Liu.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Chojnacka K. Bioaccumulation of Cr(III) ions by blue green-alga Spirulina sp. Part I. A comparison with biosorption. Am. J. Agric. Biol. Sci. 2007;2:218–223. doi: 10.3844/ajabssp.2007.218.223. [DOI] [Google Scholar]
- 2.Mehta S.K., Gaur J.P. Use of algae for removing heavy metal ions from wastewater: Progress and prospects. Crit. Rev. Biotechnol. 2005;25:113–152. doi: 10.1080/07388550500248571. [DOI] [PubMed] [Google Scholar]
- 3.Chojnacka K., Wojciechowski P.M. Bioaccumulation of Cr(III) ions by blue green-alga Spirulina sp. Part II. Mathematical modeling. Am. J. Agric. Biol. Sci. 2007;2:291–298. doi: 10.3844/ajabssp.2007.291.298. [DOI] [Google Scholar]
- 4.Krejci M.R., Wasserman B., Finney L., McNulty I., Legnini D., Vogt S., Joester D. Selectivity in biomineralization of barium and strontium. J. Struct. Biol. 2011;176:192–202. doi: 10.1016/j.jsb.2011.08.006. [DOI] [PubMed] [Google Scholar]
- 5.Bayramoğlu G., Yakup Arica M. Construction a hybrid biosorbent using Scenedesmus quadricauda and Ca-alginate for biosorption of Cu(II), Zn(II) and Ni(II): Kinetics and equilibrium studies. Bioresour. Technol. 2009;100:186–193. doi: 10.1016/j.biortech.2008.05.050. [DOI] [PubMed] [Google Scholar]
- 6.Romera E., González F., Ballester A., Blázquez M.L., Muñoz J.A. Biosorption with algae: A statistical review. Crit. Rev. Biotechnol. 2006;26:223–235. doi: 10.1080/07388550600972153. [DOI] [PubMed] [Google Scholar]
- 7.Zhang J.Y., Ding T.D., Zhang C.L. Biosorption and toxicity responses to arsenite (As[III]) in Scenedesmus Quadricauda. Chemosphere. 2013;92:1077–1084. doi: 10.1016/j.chemosphere.2013.01.002. [DOI] [PubMed] [Google Scholar]
- 8.Jena M., Adhikary S.P. Chlorococcales (Chlorophyceae) of eastern and north-eastern states of India. Algae. 2007;22:167–183. doi: 10.4490/ALGAE.2007.22.3.167. [DOI] [Google Scholar]
- 9.Reavie E.D., Jicha T.M., Angradi T.R., Bolgrien D.W., Hill B.H. Algal assemblages for large river monitoring: Comparison among biovolume, absolute and relative abundance metrics. Ecol. Indic. 2010;10:167–177. doi: 10.1016/j.ecolind.2009.04.009. [DOI] [Google Scholar]
- 10.Terry P.A., Stone W. Biosorption of cadmium and copper contaminated water by Scenedesmus abundans. Chemosphere. 2002;47:249–255. doi: 10.1016/S0045-6535(01)00303-4. [DOI] [PubMed] [Google Scholar]
- 11.Zhang H., Hu H.J., Chao A.M., Xie W.F., Cen J.Y., Lü S.H. Seasonal changes of phytoplankton community structure in Jinshuitan Reservoir, Zhejiang, China. Acta Ecol. Sin. 2013;33:944–956. doi: 10.5846/stxb201205020630. [DOI] [Google Scholar]
- 12.Hu Z.L., Wong Y.S., Tam F.Y. Bioaccumulation of nickel by various Scenedemus species in culture solution containing nickel. Acta Bot. Sin. 2002;44:978–982. [Google Scholar]
- 13.Omar H.H. Bioremoval of zinc ions by Scenedesmus obliquus and Scenedesmus quadricauda and its effect on growth and metabolism. Int. biodeterior. Biodegrad. 2002;50:95–100. doi: 10.1016/S0964-8305(02)00048-3. [DOI] [Google Scholar]
- 14.Chong A.M.Y., Wong Y.S., Tam N.F.Y. Performance of different microalgal species in removing nickel and zinc from industrial wastewater. Chemosphere. 2000;41:251–257. doi: 10.1016/S0045-6535(99)00418-X. [DOI] [PubMed] [Google Scholar]
- 15.Fargašová A., Bumbálová A., Havránek E. Metal bioaccumulation by the freshwater alga Scenedesmus quadricauda. J. Radioanal. Nucl. Chem. 1997;218:107–110. [Google Scholar]
- 16.Awasthi M., Rai L.C. Interactions between zinc and cadmium uptake by free and immobilized cells of Scenedesmus quadricauda (Turp.) Breb. Acta Hydrochim. Hydrobiol. 2006;34:20–26. doi: 10.1002/aheh.200400607. [DOI] [Google Scholar]
- 17.Liu M.X., Dong F.Q., Yan X.Y., Zeng W.M., Hou L.Y., Pang X.F. Biosorption of uranium by Saccharomyces cerevisiae and surface interactions under culture conditions. Bioresour. Technol. 2010;101:8573–8580. doi: 10.1016/j.biortech.2010.06.063. [DOI] [PubMed] [Google Scholar]
- 18.Zhao H.H. Master’ Thesis. Nanjing University of Technology; Nanjing, China: Jun 30, 2002. Study on Large Volume Poured Cement Based Low and Intermediate Level Radioactive Waste and Mechanism of Cs Solidified. [Google Scholar]
- 19.Liu M.X., Pang X.F., Dong F.Q., Zhang W., Hou L.Y. The Ashing and Volume Decreasing Analysis of Biosorption of Strontium by Yeast (Saccaromyces cerevisiae) Cell; Proceedings of the 4th International Conference on Bioinformatics and Biomedical Engineering (iCBBE); Chengdu, China. 18–20 June 2010. [Google Scholar]
- 20.Vallarta R., Jr., Cao E., Sibal Y.R. Cadmium uptake in Synechococcus aquatilis (Reynaud) strain SY01. Sci. Diliman. 1998;10:39–46. doi: 10.4197/Sci.10-1.4. [DOI] [Google Scholar]
- 21.Li M., Xu J., Liu Z.L., Xu J. Strontium stress on marine microalgae Dicratetia inornata growth and antioxidant enzymes activity. Oceanol. Limnol. Sin. 2004;35:467–471. [Google Scholar]
- 22.Li M., Xie X.T., Xue R.H., Liu Z.L. Effects of strontium-induced stress on marine microalgae Platymonas subcordiformis (Chlorophyta: Volvocales) Chin. J. Oceanol. Limnol. 2006;24:154–162. doi: 10.1007/BF02842815. [DOI] [Google Scholar]
- 23.Liu J., Sheng H.-J., Xu Y.-Q., Feng K. Effect of Fe on the growth of Scenedesmus quadricauda. Environ. Pollut. Control. 2008;30:61–64. (in Chinese) [Google Scholar]
- 24.Gipps J.F., Coller B.A.W. Effect of physical and culture conditions on uptake of cadmium by Chlorella Pyrenoidosa. Aust. J. Mar. Freshwater Res. 1980;31:747–755. doi: 10.1071/MF9800747. [DOI] [Google Scholar]
- 25.Dai S.-J., Gao T., Wang Y.-J., Liu W.-G. The Influence of co-existing ions on adsorping cadmium in water by water-washing waste Saccharomyces Cerevisiae. Non-Ferr. Min. Metall. 2008;24:79–80. (in Chinese) [Google Scholar]
- 26.Jiang B.H., Lin B.Q. Adsorption and absorption of nickel by Ankistrodesmus sp. and effect of nickel on morphlogical structure of its cell. Chin. J. Appl. Environ. Biol. 2000;6:535–541. [Google Scholar]
- 27.Davis T.A., Volesky B., Mucci A. A review of the biochemistry of heavy metal biosorption by brown algae. Water Res. 2003;37:4311–4330. doi: 10.1016/S0043-1354(03)00293-8. [DOI] [PubMed] [Google Scholar]
- 28.Wu H.S., Zhang H.L., Zhang A.Q. Biosorption on heavy metals by Chelorelia. Environ. Chem. 2004;23:173–177. doi: 10.1897/02-652. [DOI] [Google Scholar]
- 29.Tu J., Zhang L., Zhao L., Yu Y.T. The biosorption of heavy metal ions from wastewater by non-living Rhizopus nigricans. Environ. Sci. 1994;16:12–15. [Google Scholar]
- 30.Ruggiero C.E., Boukhalfa H., Forsythe J.H., Lack J.G., Hersman L.E., Neu M.P. Actinide and metal toxicity to prospective bioremediumtion bacteria. Environ. Microbiol. 2005;7:88–97. doi: 10.1111/j.1462-2920.2004.00666.x. [DOI] [PubMed] [Google Scholar]
- 31.Macfie S.M., Welbourn P.M. The cell wall as a barrier to uptake of metal ions in the unicellular green alga Chlamydomonas reinhardtii (Chlorophyceae) Arch. Environ. Contam. Toxicol. 2000;39:413–419. doi: 10.1007/s002440010122. [DOI] [PubMed] [Google Scholar]
- 32.Fan J.L., Wei X.Z., Wan L.C., Zhang L.Y., Zhao X.Q., Liu W.Z., Hao H.Q., Zhang H.Y. Disarrangement of actin filaments and Ca2+ gradient by CdCl2 alters cell wall construction in Arabidopsis thaliana root hairs by inhibiting vesicular trafficking. J. Plant Physiol. 2011;168:1157–1167. doi: 10.1016/j.jplph.2011.01.031. [DOI] [PubMed] [Google Scholar]
- 33.Latha J.N.L., Rashmi K., Mohan P.M. Cell-wall-bound metal ions are not taken up in Neurospora crassa. Can. J. Microbiol. 2005;51:1021–1026. doi: 10.1139/w05-096. [DOI] [PubMed] [Google Scholar]
- 34.Li F., Zhang X.P., Huang K., Xiang Z., Wang E.S. Biosorption of copper cation by Candida utilis cells and cell walls. J. Chang. Coll. 1999;11:62–64. [Google Scholar]
- 35.Texier A.C., Andrès Y., Illemassene M., Le Cloirec P. Characterization of lanthanide ions binding sites in the cell wall of Pseudomonas aeruginosa. Environ. Sci. Technol. 2000;34:610–615. doi: 10.1021/es990668h. [DOI] [Google Scholar]
- 36.Gupta V.K., Rastogi A. Sorption and desorption studies of chromium(VI) from nonviable cyanobacterium Nostoc muscorum biomass. J. Hazard. Mater. 2008;154:347–354. doi: 10.1016/j.jhazmat.2007.10.032. [DOI] [PubMed] [Google Scholar]
- 37.Langmuir I. The adsorption of gases on plane surfaces of glass, mica and platinum. J. Am. Chem. Soc. 1918;40:1361–1403. [Google Scholar]
- 38.Freundlich H.M.F. Über die adsorption in lösungen. Z. Phys. Chem. 1906;57:385–470. [Google Scholar]
- 39.Koble R.A., Corrigan T.E. Adsorption isotherms for pure hydrocarbons. Ind. Eng. Chem. 1952;44:383–387. doi: 10.1021/ie50506a049. [DOI] [Google Scholar]
- 40.Yuvaraja G., Krishnaiah N., Subbaiah M.V., Krishnaiah A. Biosorption of Pb(II) from aqueous solution by Solanum melongena leafpowder as a low-cost biosorbent prepared from agricultural waste. Colloids Surf. B: Biointerfaces. 2014;114:75–81. doi: 10.1016/j.colsurfb.2013.09.039. [DOI] [PubMed] [Google Scholar]
- 41.Desta M.B. Batch sorption experiments: Langmuir and Freundlich isotherm studies for the adsorption of textile metal ions onto Teff Straw (Eragrostis tef ) agricultural waste. J. Thermodyn. 2013;2013 doi: 10.1155/2013/375830. [DOI] [Google Scholar]
- 42.Nagy B., Măicăneanu A., Indolean C., Mânzatu C., Silaghi-Dumitrescu L., Majdik C. Comparative study of Cd(II) biosorption on cultivated Agaricus bisporus and wild Lactarius piperatus based biocomposites. Linear and nonlinear equilibrium modelling and kinetics. J. Taiwan Inst. Chem. E. 2014;45:921–929. doi: 10.1016/j.jtice.2013.08.013. [DOI] [Google Scholar]
- 43.Ergene A., Ada K., Tan S., Katırcıoğlu H. Removal of Remazol Brilliant Blue R dye from aqueous solutions by adsorption onto immobilized Scenedesmus quadricauda: Equilibrium and kinetic modeling studies. Desalination. 2009;249:1308–1314. doi: 10.1016/j.desal.2009.06.027. [DOI] [Google Scholar]
- 44.Ho Y.S., McKay G. Sorption of dye from aqueous solution by peat. Chem. Eng. J. 1998;70:115–124. doi: 10.1016/S0923-0467(98)00076-1. [DOI] [Google Scholar]
- 45.Ho Y.S., McKay G. The kinetics of sorption of divalent metal ions onto Sphagnum moss peat. Water Res. 2000;34:735–742. doi: 10.1016/S0043-1354(99)00232-8. [DOI] [Google Scholar]
- 46.Agarry S.E., Ogunleye O.O., Aworanti O.A. Biosorption equilibrium, kinetic and thermodynamic modelling of naphthalene removal from aqueous solution onto modified spent tea leaves. Environ. Technol. 2013;34:825–839. doi: 10.1080/09593330.2012.720616. [DOI] [PubMed] [Google Scholar]
- 47.Ho Y.S. Citation review of Lagergren kinetic rate equation on adsorption reactions. Scientometrics. 2004;59:171–177. doi: 10.1023/B:SCIE.0000013305.99473.cf. [DOI] [Google Scholar]
- 48.Ho Y.S. Review of second-order models for adsorption systems. J. Hazard. Mater. 2006;136:681–689. doi: 10.1016/j.jhazmat.2005.12.043. [DOI] [PubMed] [Google Scholar]
- 49.Argüello J.M. Identification of ion-selectivity determinants in heavy-metal transport P1B-type ATPases. J. Membr. Biol. 2003;195:93–108. doi: 10.1007/s00232-003-2048-2. [DOI] [PubMed] [Google Scholar]
- 50.Argüello J.M. Ph.D. Thesis. Worcester Polytechnic Institute; Worcester, MA, USA: Dec 30, 2006. HMA2. A Transmembrane Zn2+ Transporting ATPase from Arabidopsis thaliana. [Google Scholar]