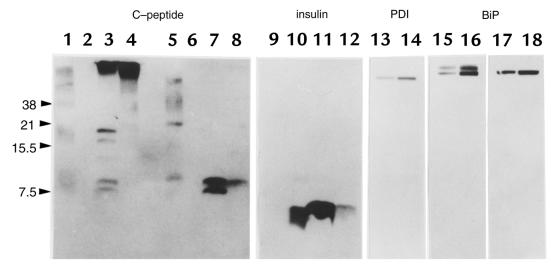
Figure 7.
Immunoblotting analysis of the islet proteins. An equal amount of the islet protein (50 μg) from either C57BL/6J (lanes 3 and 7) or Mody mice (lanes 4 and 8) was loaded in each lane onto 16.5% polyacrylamide gel with (lanes 5–8) or without (lanes 1–4) 100 mM DTT. Human proinsulin (lanes 1 and 5) and human insulin (lanes 2 and 6) were loaded as standards. The human C-peptide ran off this tricine–SDS-PAGE system (data not shown). Immunoblotting was performed using anti–C-peptide antibodies (lanes 1–8). On the same membranes, similar analyses were performed using anti-insulin (lanes 9–12, corresponding to lanes 1–4 in the C-peptide immunoblot), anti-PDI (lanes 13 and 14, corresponding to lanes 3 and 4), and anti-BiP antibodies (lanes 15–18, corresponding to lanes 3, 4, 7, and 8). The islet protein lysed by either sample buffer containing 3% SDS (16) or acid-ethanol (26) revealed similar proteins immunoreactive to anti–C-peptide antibodies on nonreducing gels (data not shown). DTT, dithiothreitol; PDI, protein disulfide isomerase.