Abstract
Prevalence of overweight and obesity has risen in the United States over the past few decades. Concurrent with this rise in obesity has been an increase in pregravid body mass index and gestational weight gain affecting maternal body composition changes in pregnancy. During pregnancy, many of the assumptions inherent in body composition estimation are violated, particularly the hydration of fat-free mass, and available methods are unable to disentangle maternal composition from fetus and supporting tissues; therefore, estimates of maternal body composition during pregnancy are prone to error. Here we review commonly used and available methods for assessing body composition changes in pregnancy, including: (1) anthropometry, (2) total body water, (3) densitometry, (4) imaging, (5) dual-energy X-ray absorptiometry, (6) bioelectrical impedance and (7) ultrasound. Several of these methods can measure regional changes in adipose tissue; however, most of these methods provide only whole-body estimates of fat and fat-free mass. Consideration is given to factors that may influence changes in maternal body composition, as well as long-term maternal and offspring outcomes. Finally, we provide recommendations for future research in this area.
INTRODUCTION
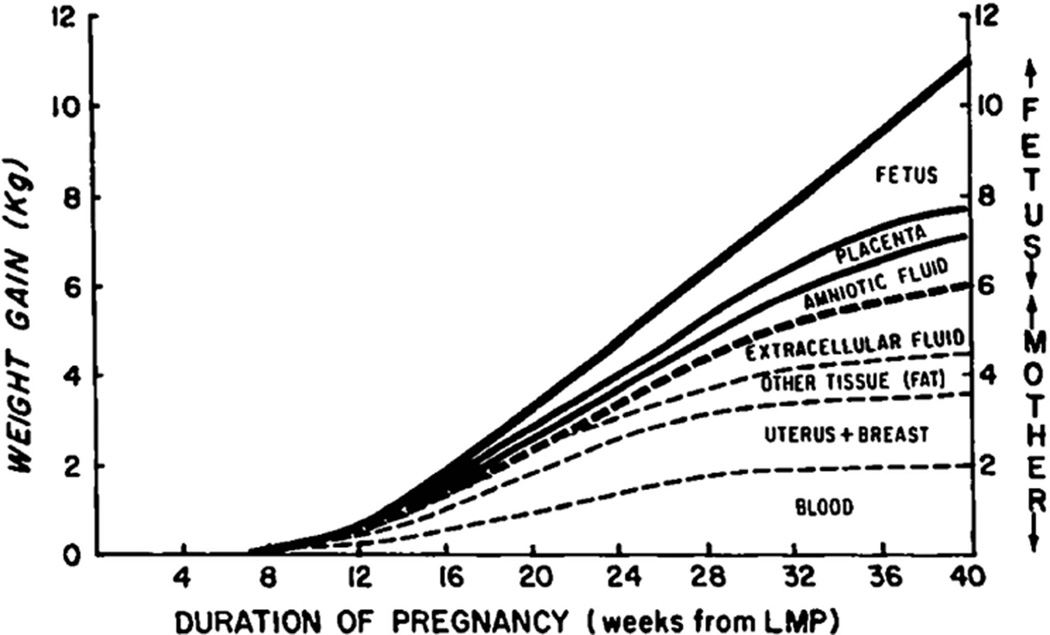
The body exhibits dynamic changes in composition during pregnancy to support the fetus as it develops from conceptus to live born infant. These changes are reflected in gestational weight gain (GWG), which includes gains in maternal and fetal fat mass (FM) and fat-free mass (FFM), as well as the placenta and amniotic fluid (Figure 1). The Institute of Medicine GWG guidelines by prepregnancy body mass index (BMI) aim to optimize maternal, fetal and infant health outcomes and further recommend that women achieve a healthy body weight before pregnancy.1 Prevalence of overweight/obesity in women of childbearing age remains high and, moreover, over half of women recently have gained excessive weight in pregnancy with consequences for the mother and offspring.2 With excess GWG, mothers are at increased risk of cesarean delivery3 and may be at increased risk of abnormal glucose metabolism and pregnancy-induced hypertension.4 Furthermore, offspring are at risk of high birth weight,4 macrosomia,4 large-for-gestational age,3,4 impaired fetal growth4 and preterm birth.3,4 Postpartum, mothers with excessive GWG are at risk for weight retention,4 subsequent obesity4 and likely obesity-associated health consequences, including type 2 diabetes and cardiovascular disease thereafter, but evidence is limited in this area.1,5,6 Offspring of mothers with excessive GWG have higher weight-for-age Z-scores and length-for-age Z-scores in infancy,7 higher BMI Z-scores in childhood8 and possibly a greater risk of obesity-associated sequelae.5,9
Figure 1.
Pattern and components of average weight gain in pregnancy. LMP, last menstrual period. Source: Pitkin RM. Nutritional support in obstetrics and gynecology. Clin Obstet Gynecol 1976; 19: 489–513. Reprinted.
Cumulatively, these adverse health consequences from excessive GWG may pose an even greater threat to maternal and infant long-term health in resource-poor settings undergoing various phases of the nutrition transition.10 The nutrition transition is marked by shifts in diet from traditional foods to a more Western-type diet along with decreasing physical activity that propagate obesity and nutrition-related non-communicable diseases, such as cardiovascular disease and diabetes.11 As women of reproductive age in these settings may have been previously exposed to undernutrition and are now becoming overweight/ obese, excessive GWG among mothers previously exposed to undernutrition may further lead toward heightened risk of maternal and offspring obesity and nutrition-related diseases; however, evidence is limited in this area.
Previously, various components of GWG, including total body water (TBW), FM and FFM—where TBW was estimated by deuterium dilution; and FM and FFM estimated with a four-compartment model (details later in this review)—were found to be positively correlated with total GWG;12 but only FM gain was related to initial BMI values.12 Higher initial BMI was associated with greater FM gains.12 GWG and FM gains were correlated with fat retention postpartum, while TBW and FM gains were correlated with infant birth weight.12 Although several studies have examined how GWG relates to maternal and infant health outcomes,5,7,12,13 there is much less evidence related to the association between change in maternal body composition and maternal and infant short- and long-term health which may be due to measurement challenges in this population.
Measuring maternal body composition during gestation is challenged by available in vivo measuring methods that cannot differentiate between maternal and fetal depots14 and approach the maternal–fetal dyad as a single unit. Moreover, some pregnancy-induced changes in body composition violate the assumptions that are the foundation of many of the commonly available measurement methods, and pregnancy-specific corrections (that often vary by gestational age) are needed. For example, TBW increases during pregnancy by about 5–8 liters15–18 and the composition of lean tissue changes as pregnancy progresses, thereby invalidating a basic assumption that underlies many measurement techniques, that 73% of the adult’s FFM compartment is water.19–22 In reality, obtaining an accurate estimate of pregravid weight status and/or body composition immediately before or early in pregnancy is not feasible, thereby affecting the validity of body composition change estimates. The Institute of Medicine guidelines assume that women gain on average 0.5–2 kg in the first trimester according to their pregravid BMI in their recommendations for total GWG;1 however, evidence suggests that the pattern of GWG in this period is highly variable. For example, total first trimester GWG was − 0.4, 2.7 and 6.9 kg at the 10, 50 and 90th percentiles, respectively, in predominately non-Hispanic white women from the United States.23 This GWG variability also may reflect changes in body composition; therefore, in order to accurately estimate change in composition, a measurement of pregravid body composition in close proximity to conception is essential.
Here we review the state of the literature with regard to how maternal body composition changes during pregnancy. Methods for measuring maternal body composition are reviewed. Strengths and limitations of each method are discussed. Previous research is summarized for each component of body composition, including changes in body weight or mass, FM, body water compartments and bone mineral density. An overview of specific factors influencing maternal body composition during pregnancy are discussed, including initial weight status, parity, race/ethnicity, genetics and pregnancy in adolescent period. Finally, we briefly review current evidence relating maternal body composition during pregnancy to maternal and infant long-term health and provide recommendations for future research in this area.
SEARCH STRATEGY
Sections on maternal body composition from the Institute of Medicine recommendations for GWG and several textbooks were consulted.1,14,16,24–26 PubMed searches up to July 2013 with the terms ‘body composition changes pregnancy’ or ‘body water changes pregnancy’ or ‘adipose tissue changes pregnancy’ were conducted with limits of human studies and English language, identifying 507 articles, excluding duplicates. Additional PubMed searches were conducted by (1) adding key terms for each assessment method and (2) identifying predictors and outcomes of body composition changes in pregnancy. Papers or abstracts were reviewed when the study (1) focused on changes in body composition across the course of pregnancy or from prepregnancy to postpartum; and (2) focused on predictors and outcomes of body composition changes. Further, reference lists of these papers were reviewed for additional relevant publications. Seminal papers related to the development and validation of assessment methods were also reviewed. Overall, the search process resulted in 87 sources that were synthesized and included in this review.
TECHNIQUES FOR MEASURING MATERNAL BODY COMPOSITION
Table 1 summarizes methods for estimating changes in body composition in pregnancy. Most methods theoretically divide the body into compartments from which an estimate of FM is derived. The two-compartment model divides the body into FM and FFM, while the three-compartment model further sub-divides the FFM compartment into water and a combination of mineral and protein. The four-compartment models further subdivide the FFM compartment into mineral, water and protein. The Institute of Medicine has indicated that these models are ‘satisfactory’ for estimating body composition changes in pregnancy, given that corrected values for hydration and density of FFM are applied, and for three- and four-compartment models that FFM hydration or density are measured.1 Furthermore, the Food and Agriculture Organization, World Health Organization and the United Nations University have also issued a similar joint statement regarding ‘acceptability’ of these models if appropriate corrections are applied.27 These corrections and measures will be discussed later in this review.
Table 1.
Summary of methods to measure body composition changes in pregnancy
| Method | Participant complexity |
Technical complexity |
Source of Error | Compartment model |
Regional distribution | Suitable in field settings |
|---|---|---|---|---|---|---|
| Anthropometry—skinfold thickness | Simple | Simple | Measurement error—requires technician expertise. Over the course of pregnancy, changes in (1) compressibility of skinfold thickness, edema and (3) ability to measure certain regions Equations to estimate total body fat available for only certain gestational ages | 2 | Yes | Y |
| Total body water (TBW) | Simple | Specialized instruments | Hydration of fat-free mass. Unable to disentangle the maternal–fetal unit | 2/Multiplea | No | Y |
| Underwater weighing | Complex | Specialized instruments | Unable to disentangle the maternal–fetal unit | 2/Multiplea | No | N |
| Air displacement plethysmography (ADP/BodPod) | Complex | Specialized instruments | Unable to disentangle the maternal–fetal unit | 2/Multiplea | No | N |
| Magnetic resonance imaging (MRI) | Simple | Specialized instruments | Methods have not been developed specifically for pregnancy; therefore, the source of error is not fully understood | 2 | Yes | N |
| Bioelectrical impedance analysis (BIA) | Simple | Specialized, Portable Equipment Depending on model | Has not been validated against reference methods in pregnancy. Unable to disentangle the maternal–fetal unit | 2 | Yes, but not validated | Y |
| Dual energy X-ray absorptiometry (DEXA) | Simple—for use in prenatal and postnatal | Specialized instrument | Not suitable during pregnancy due to radiation exposure. Bone mineral content estimates used for four-compartment models. Fat and fat-free mass estimates prone to error due to changes in the composition of lean tissue that may persist into the postpartum period | 2/Multiplea | Yes, but not validated | N |
| Ultrasound | Simple | Specialized instrument, available in most hospital settings | Estimates regional adiposity, not total body composition. Has not been validated against reference methods in pregnancy | Yes, but not validated | N |
These methods are used for multi-component models in combination with other body composition measurements and body mass.
Anthropometry
Anthropometric measurements, particularly skinfold thicknesses (SFT) and mid-upper arm circumferences, have been used extensively to estimate changes in body composition in pregnancy. Typically FM changes are estimated using equations with body weight, SFT and often circumference measures. Although several equations to estimate FM using anthropometric measurements have been developed in non-pregnant women,28–31 these equations overestimate fat changes in pregnancy when compared with estimates from a four-compartment model.22 Paxton et al.22 developed and validated equations for estimating fat change from 14 to 37 weeks of gestation and an equation to predict FM at 37 weeks22 (Table 2). Similarly, Huston Presley et al.32 developed and validated an equation for estimating FM at 30-week gestation derived from SFT measures and weight (Table 2). These equations, however, are for specific gestational ages and may not apply to other times in pregnancy. Thus use of SFT measurements themselves rather than estimates of fat and FFM are often preferred.
Table 2.
Equations for estimating body fat in pregnancy
| Anthropometric equations | Population |
| Paxton (1) fat change from 14 to 37 weeks: | White, black and Hispanic women in New York City, NY, USA |
| Fat change, kg = 0.77 (weight change, kg) +0.07 (change in thigh skinfold thickness, mm) - 6.13. | |
| Paxton (1) body fat mass at term (37 weeks): | |
| Fat mass, kg = 0.40 (weight at week 37, kg) + 0.16 (biceps skinfold thickness at 37 wk, mm)− 0.15 (thigh skinfold thickness at 37 weeks, mm)- 0.09 (wrist circumference at 37 weeks, mm)+0.10 (prepregnancy weight, kg) −6.56 (2). | |
| Huston Presley (2) body fat mass at 30 weeks: | Predominantly white women in Cleveland, OH, USA |
| Fat mass, kg = 0.33529 (weight, kg) +0.65664 (triceps skinfold thickness, mm)‒0.4373 (subscapular skinfold thickness, mm)+0.43461 (suprailliac skinfold thickness, mm)‒13.0538. | |
| Equations for deriving arm fat area (AFA) and arm muscle area (AMA) (3) | |
| AMA (cm2) = [mid-upper arm circumference-(triceps skinfold×π)]2/4π | |
| AFA (cm2)=(mid-upper-arm circumference2/4π -arm muscle area) (3) | |
| Equation for body composition from body density, total body water and body weight | |
| Siri (4, 5) equation for body composition: | |
| Fat mass, kg = [2.118 × (BV/body weight)] − (0.78 × TBW/body weight) − 1.354] × 100 |
Abbreviations: BV, body volume (l); TBW, total body water (kg).
Skinfold measurements and circumferences have been widely used to estimate changes in subcutaneous fat, as well as arm muscle area and arm fat area (Table 2), indicators of muscle mass and FM, in pregnancy.33–37 Several studies have described longitudinal changes in SFT in various populations of well-nourished and undernourished women.25,33–36 Together, these reports suggest highly variable changes in SFT across measurement sites over the course of pregnancy. In well-nourished populations, one study reported decreases in SFT (triceps, thigh and subscapular) from prepregnancy to 6-week gestation.36 This report and others indicate increases in SFT across several sites up to 30–35 weeks33,36 followed by few changes to 38 weeks (excluding mid-thigh skinfold that continues to increase33). Thereafter, rapid decreases occur postpartum that are likely attributable to changes in hydration following delivery rather than marked changes in subcutaneous adipose tissue. In marginally undernourished women, average triceps and subscapular SFT increased in the first and second trimester and decreased markedly in the third trimester, resulting in a net loss in skinfolds over the course of pregnancy.34 Although SFT increases were predominate and associated with GWG, declines in triceps and subscapular SFT were also observed, particularly in women who began pregnancy with more subcutaneous fat.34 In both well-nourished and undernourished women, arm fat area typically increases early in pregnancy and declines in the last trimester, while arm muscle area increases in this period.25,33,35
Estimates of body fat changes derived from skinfolds are prone to measurement error, especially during pregnancy.38 First, extensive technician training is necessary to obtain valid and reliable measures of SFT and anthropometry. Second, SFT is influenced by the compressibility of the subcutaneous adipose tissue layer that is affected by site, age, gender, recent weight changes and also pregnancy. There is some evidence that skinfolds compressibility increases in the first trimester and gradually increases thereafter in the second and third trimesters, which is believed to be attributable to the increases in hydration of extracellular components of connective tissues due to shifts in hormonal milieu.39 This study was completed in a cohort of primaparous women in the 1960s; whether these reported compressibility changes across pregnancy vary by GWG, rate of weight gain or other factors is unknown, and more research on this subject in contemporary cohorts is needed. In contrast to previous findings, percentage of skinfold compression calculated from calipers and ultrasound measurements in pregnant adolescents was found to decrease between ~11- and ~30-week gestation in the trunk region, but no changes were observed in the limb regions.40 It is important to note that these findings may not be generalizable to adult populations, as body composition changes are different in adolescent pregnancy compared with adult women,41 and whether skinfold compressibility is different in adolescent pregnancy compared with adult pregnancy has not been reported.41 Moreover, as pregnancy progresses it becomes difficult to obtain skinfold measurements from the trunk region.42 Edema may also affect the ability to obtain accurate measurements, especially in the leg region. Finally, equations have not been developed for estimating body composition changes in FM and FFM throughout pregnancy from SFT measures. Applying equations from non-pregnant populations to estimate FM and FFM may not be appropriate, particularly in resource-poor settings.
TBW
During pregnancy, TBW changes are highly variable. Several studies have reported TBW accretion of approximately 5–8 l over the course of pregnancy.15–18 However, in Swedish women TBW accretion of 6.6 l was reported by 32-week gestation,43 which is somewhat higher than previously reported taking into account the timing of this measurement relative to delivery, and therefore may indicate greater TBW accretion in pregnancy compared with previous estimates in this population.
TBW is typically measured using the dilution principle with isotope-labeled water labeled with deuterium (2H2O) or Oxygen 18 (18O), which provides an estimate of TBW in the combined maternal and fetal unit. Before administering the labeled water, a physiological baseline sample of serum, urine or saliva is collected. A precisely weighed dose of labeled water is then ingested orally or administered subcutaneously. Two methods are utilized to determine TBW by dilution: plateau and back extrapolation. With the plateau method, a second physiological sample is obtained after an equilibration period; extended in pregnancy to 4 h for oral dosing and 3 h for subcutaneous dosing to ensure equilibrium,44 as extracellular water compartments are expanded. With back extrapolation, physiological samples are collected at several intervals up to 14 days after dose administration, and TBW is calculated by back extrapolation to the time of dose.
Body composition estimates can be determined from TBW coupled with measurements of body weight and density; yet, inherent in this estimate is the assumption of the hydration of FFM, calculated as the ratio of TBW to FFM, to be approximately 0. 73.45 FFM hydration changes in pregnancy leading to errors in body fat estimates.43 Moreover, FFM hydration variability also changes during the course of pregnancy with greater variability early in pregnancy (14 weeks) compared with variability at 32 weeks,43 suggesting the two-compartment model is less accurate in individuals in early pregnancy due to heightened biological variability of the hydration of FFM. Several studies have predicted19,21,46 or estimated20,43 hydration factors which indicate that the hydration of FFM generally increases as pregnancy progresses and subsequently decreases postpartum, and correction factors are available for varying gestational ages (Table 3). These were developed in predominantly normal BMI populations from United States or Europe who were of White, Black or Hispanic origins (if reported). Because of variation in FFM hydration in severe obesity and in racially and ethnically diverse samples, these hydration factors may not apply to all populations; thus additional research is needed in this area. Another consideration is the proportion of gain in FFM relative to pregravid FFM; Van Raaij et al.19 suggested that the gain in FFM relative to prepregnancy FFM may influence the hydration of FFM which would therefore result in errors based on hydration factors calculated for a reference woman. However, based on their estimates for a range of ratios for gain in FFM relative to prepregnant value, this is unlikely to result in a substantial error, as the body fat estimates from their derivations for those with extreme ratios only differed slightly from estimates of the reference women (0.3–0.6 kg),25 and thus the authors suggest that these hydration ratios may be appropriate for most pregnant women.19
Table 3.
| Prepregnancy | Weeks |
Postpartum | Density component | Population | ||||
|---|---|---|---|---|---|---|---|---|
| 10–14 | ~ 20 | 30–32 | 35–40 | |||||
| Hydration (%) Longitudinal studies | ||||||||
| van Raaij et al.19 c | ||||||||
| No/only leg edema | 0.725 | 0.732 | 0.740 | 0.750 | Derived by van Raaij et al.19 from data on chemical analysis of six adult humans (five male, one female), BMI not reported84 | |||
| Edema | 0.725 | 0.734 | 0.748 | 0.765 | ||||
| Fidanza et al.21 c | 0.738 | 0.738 | 0.752 | 0.761 | Derived from reference body85 (based on three male cadavers): mean BMI: 20.9 kg/m2 | |||
| Lof et al.43 | 0.718 | 0.723 | 0.747 | 0.747 | 0.732 | Swedish women (n = 19), pregravid BMI: 24.2 kg/m2 | ||
| Paxton et al.22 | 0.738 | 0.757 | US women (n = 200): 55% Hispanic; 21% non-Hispanic White; 25% non-Hispanic Black; pregravid BMI: underweight (<19.8): 10.5%; normal (19.8–26.0): 61.5%; overweight (>26–29.0): 14.5%; obese (>29): 13.5% | |||||
| Sohlström and Forsum42 d | 0.669 ± 3.3e | 0.697 ± 5.0e | Swedish women (n = 16); pregravid BMI: 23.7 kg/m2 | |||||
| Hopkinson et al.48 | 0.76 ± 0.02 | 0.75± 0.02 | US women (n = 56); pregravid BMI: 22.8 kg/m2 | |||||
| Cross-sectional studies | ||||||||
| Prentice et al.46 | 0.768 | British women (n = 8); pregravid BMI: 23.1 kg/m2 | ||||||
| Catalano et al.20 | 0.762 | Predominately white US women (n = 20); pregravid BMI: 25.7 kg/m2 | ||||||
| Density (g/cm3) | ||||||||
| Paxton et al.22 f | 1.100 | 1.091 | Maternal and fetal | Estimates based on the Selinger four-compartment model;86 population details above | ||||
| Fidanza et al.21 c | 1.100 | 1.100 | 1.097 | 1.092 | 1.087 | Maternal and fetal | Derived from reference body (based on three male cadavers) and based on Hytten’s report:25 mean BMI: 20.9 kg/m2 | |
| Hopkinson et al.48g | 1.087 ± 0.006 | 1.094 ±0.007 | Calculated maternal | Estimates based on the Fuller four-compartment model corrected according to the estimates by van Raaij et al. at 36 weeks;19 US women (n = 56); pregravid BMI: 22.8 kg/m2 | ||||
| Density (g/l) of fat-free mass gain | ||||||||
| van Raaij et al.19 c, f | ||||||||
| No/only leg edema | 1.022 | 1.027 | 1.032 | 1.030 | Maternal and fetal | Estimates derived from Hytten, population details not reported | ||
| Edema | 1.022 | 1.020 | 1.023 | 1.019 | ||||
Hydration factor for fat-free mass calculated as total body water/ fat-free mass.
Values reported are mean ± s.d.
Estimates derived from Hytten’s estimates of composition of tissues.25
Fat-free mass estimated with magnetic resonance imaging (MRI).
Because fat-free mass was estimated with MRI, these values are based on different assumptions and are not comparable with the other values in the table.
Density estimated with underwater weighing and estimated using the Goldman and Buskirk equation.87
Derived from equations from Van Raaij et al. (1988) developed according to the Hytten’s estimates of composition of tissues.25
Although TBW measurements using stable isotope methods are considered safe in pregnancy, women may not want to ingest the stable isotope, and the method may be difficult to perform in settings without access to refrigeration. Other indirect methods of measuring body water in pregnancy have not been developed or validated against reference methods across a range of gestational ages (for example, TBW estimates from bioelectrical impedance analysis (BIA) or bioimpedance spectroscopy (BIS)); while BIS TBW estimates were comparable to deuterium estimates at 14 weeks, BIS TBW estimates were significantly lower than deuterium at 32 weeks, indicating that a revised model is needed for this period.47 Moreover, the correction factors for TBW estimates needed to derive body composition estimates may need to be population specific (normal weight, overweight, obese).
Densitometry
Body density can be estimated using hydrodensitometry (HD), otherwise known as underwater weighing, or air-displacement plethysmography (ADP) from which estimates of body composition of the combined, maternal–fetal unit can be derived; these methods are unable to assess body density of the pregnant women independent of the fetus and supporting tissues. Body density (DB) is estimated from the ratio of body mass (M) to volume (V) (DB = M/V), from which estimates of FM and FFM can be derived incorporating assumed respective densities for fat (DF) (0.900 g/cm3) and FFM (DFFM) (variable, see Table 3): 1/DB = FM/ DF+FFM/DFFM.
Estimation of body components with these methods is affected by the shifts in the density and composition of FFM over the course of pregnancy. Initially FFM increments are predominately maternal tissues, while in later pregnancy FFM changes are predominately fetal tissues of lower density.24 As such, FFM density is decreased in late pregnancy relative to a non-pregnancy state; estimates of body composition during this time that do not account for this decrease underestimate FFM and overestimate FM. Studies have been conducted in an effort to obtain a better estimate of the density of maternal and/or the maternal–fetal unit FFM across the course of pregnancy. FFM density values have also been estimated for various gestational ages (Table 3).19,21 These values may not be applicable to all populations and research to understand if body density values vary by maternal age, race/ ethnicity and size is warranted.
HD uses Archimedes’ principle to estimate body density, where due to buoyancy the weight of a body immersed in fluid is equal to the weight of displaced fluid: body volume = (weightAIR -weightWATER)/densityWATER. Several studies have utilized HD, which has primarily been incorporated into four-compartment model estimates of body composition.12,15,48 More recently ADP has become more popular to estimate body composition and density, but use in pregnancy has been limited. ADP is based on Boyle’s law, where air compressed will decrease in volume proportional to increasing pressure at a constant temperature. ADP is measured with the BodPod (Cosmed, Concord, CA, USA), a device with two chambers, one where the subject sits of approximately 450 l and a reference chamber of approximately 300 l, and a moving diaphragm between the chambers that produces contrasting small volume and pressure changes in each chamber.49,50 Based on these changes coupled with measurement and correction for lung volume (estimated through a breathing exercise), body volume and composition (FM and FFM) are estimated. Subjects need to wear skintight clothing, such as a tight-fitting swimsuit or undergarments, to minimize the amount of trapped air in hair or clothes that would result in an overestimate of body volume.
HD and ADP are non-invasive methods to obtain body density of the maternal–fetal unit; however, these methods are not suitable for field research and require specialized equipment. Although HD has been used extensively in pregnancy, it may be difficult for pregnant women to be submerged in water, particularly later in pregnancy. Thus ADP may be the preferred body volume and density method in pregnancy for measuring the combined maternal–fetal unit due to its ease. However, as the system has not been validated in persons weighting >250 kg, obese pregnant women whose body weight exceeds this limit at any point in pregnancy, even if the system can accommodate their body size, should not be measured.
Imaging
Imaging methods, including computed tomography and magnetic resonance imaging (MRI) and three-dimensional photonic scanning (3DPS), can be utilized to estimate body composition; however, 3DPS and MRI are still in the exploratory stages for pregnancy, while computed tomography is contraindicated due to radiation exposure and has not been utilized to evaluate changes from prepregnancy to postpartum. As such, this discussion will focus on 3DPS and MRI.
MRI is the only method available for in vivo measurement of adipose tissue, skeletal muscle and organ mass that can estimate changes in mass and distribution over the course of pregnancy; however, density and hydration cannot be measured with MRI. There are no known risks to the use of MRI at low field strengths (for example, 1.5 Tesla) but its safety during the first trimester has not been sufficiently evaluated.51 There are no published studies to date that have used MRI to estimate changes in maternal body composition during pregnancy. One study used MRI before pregnancy and again 5–10 days postpartum in 15 Swedish women.52 On average, women gained 7.4 kg from prepregnancy to 1-week postpartum in total body adipose tissue.52 This included a 5.4 l gain in whole body adipose tissue of which gains were primarily subcutaneous 4.1 l gain in subcutaneous tissues and 1.3 l in non-subcutaneous tissues. There was marked variation between women in terms of the overall changes and regional distribution of the adipose tissue; moreover, a net loss in tissue volume was observed in a few women.52 Although this study provides much insight regarding gains in subcutaneous and overall body fat in pregnancy, the time period before conception was not reported. If this period was extended beyond a few weeks, error may have been introduced to the estimates of changes in body composition; however, obtaining estimates immediately before conception is typically not feasible for most researchers.
In vivo validation of MRI estimates is not feasible; therefore, studies have compared MRI estimates obtained before or after pregnancy with other body composition assessment methods.42,53 In 25 healthy Swedish women, the difference between the percentage of fat estimates from HD and MRI was 1.4 ± 2.9%, which did not vary by percentage body fat (r = 0.39, P>0.05).53 In 11–16 women, MRI estimates of percentage of fat measured before and after pregnancy were significantly lower than estimates from skinfolds and body water dilution.42 Although MRI research is currently the most cutting-edge method available for use in pregnancy, there are several limitations, including cost of the MRI test, technician expertise, unsuitable for field-based settings and time required for the test and analysis. Moreover, pregnant women may not want to be exposed to a magnetic field. Finally, overweight and obese women may have difficultly fitting in the MRI device, particularly later in pregnancy.
3DPS has been validated for measuring body volumes,54,55 circumferences, length and percentage of fat in adults;55 however, 3DPS has not been validated to estimate dimensions and composition in pregnancy. Two cross-sectional studies have examined changes in body shape in relation to parity.56,57 In British women, parity was associated with increased waist and thigh girths in women aged o41 years; but in older women this association was not observed, suggesting that effects of parity on body dimensions are attenuated over time.58 Somewhat similar regional trends were observed in Thai women aged o41 years, in whom parity was associated with greater waist and arm girth but lower calf girth.57 In contrast to the findings in British women, an association was observed between parity and increased arm girths, but reduced hip, and calf girths in older Thai women, which is likely attributable to the differences in socioeconomic status, activity and diets between these populations.57
Ultrasound
Several cross-sectional59 and longitudinal40,60 studies have used ultrasound measurements in pregnancy to measure maternal regional subcutaneous and visceral fat; however, standardized protocols for body fat assessment with ultrasound have not been developed. Thus it may be challenging to reliably and validly track adipose tissue changes over the course of pregnancy, particularly as the compressibility of tissue changes (see skinfolds section), thereby influencing ultrasound estimates. In adult men (n = 124), ultrasound and SFT estimates of subcutaneous fat at triceps, biceps, subscapular, waist, suprailliac, thigh and calf sites were significantly negatively correlated (r = − 0.48 to − 0.75, P < 0.001) with HD-derived estimates of body density.61 In non-pregnant females (n = 50), ultrasound-derived intra-abdominal thickness was correlated (r = 0.67, P < 0.001) with computed tomography estimates of visceral fat.62 Although both of these studies had significant correlations, the coefficients were indicative of mediocre or poor prediction, which is likely attributable to the various factors influencing the compressibility of the skin at the ultrasound site even in non-pregnant adults. In pregnancy, ultrasound has not been validated against a suitable reference method, such as MRI. However, because ultrasound is widely available in hospital-based settings, further research is needed to develop standard protocols for measurement and analysis if validity and reliability is demonstrated.
BIA
BIA is an inexpensive, rapid and non-invasive method for estimating body composition. BIA is based on the assumptions and relationships regarding electrical properties of various biological tissues at varying frequencies. BIA devices use an alternating current with very low amperage that uses the water content of the body as a conductor. The impedance, or opposition, of the electrical flow by tissues allows for estimation of TBW from which estimates of fat and FFM can be derived.
Several factors compromise the validity and ability to validate BIA in pregnancy. First, estimates of TBW are influenced by the ratio of intracellular (ICW) to extracellular water (ECW),63 which changes markedly throughout pregnancy compared with a non-pregnant state64 and is likely to vary between women and by gestational age. BIA devices currently estimate this ratio with manufacturer-developed regression equations; no comparisons of BIA ECW/ICW estimates to reference methods have been reported. Previously, BIS (an approach similar to BIA that measures impedance at varying frequencies) estimates of ICW, ECW and TBW at 14 weeks and ICW at 32 weeks were similar to reference methods (sodium bromide and deuterium dilution), while ECW and TBW estimates were lower than reference values at 32 weeks.47 The authors report that the model utilizing wrist-to-ankle BIS was developed in non-pregnant populations and may not be suitable for pregnancy, where greater water is located in the trunk region compared with non-pregnant populations, and therefore suggest development of a new model for BIS for assessment of body water in pregnancy.47 Therefore, it is unclear whether it is even feasible to validate BIA in pregnancy owing to the between-women variability of changes in TBW concurrent with changes in composition.
Dual-energy x-ray absorptiometry (DXA)
DXA is unsuitable in pregnancy due to radiation exposure; however, DXA is used before and after pregnancy to measure bone mineral content (BMC), which is one of the components in four-compartment model estimation of body composition,12,17,48 and can also be used for estimates of fat and FFM. Use of DXA estimates of BMC for four-compartment estimates of fat and FFM assumes no changes across the course of pregnancy and no changes from delivery to time of measurement. However, several studies have reported conflicting evidence of either no significant changes65–69 or small changes70–73 in BMC at specific skeletal sites or total body BMC. Another study reported declines at several sites and in whole-body bone mineral area density (bone mineral content adjusted for area of the skeleton scanned) in pregnancy.74 Together this indicates that further research is needed in this area. In the event that BMC values change during pregnancy, correction factors would need to be developed.
Several assumptions underlie DXA estimates of body composition for soft tissue: (1) a constant attenuation of the fat and lean tissue, (2) body thickness does not influence measurements, and (3) the fat content of the area analyzed is comparable to the fat content of the non-analyzed areas; and for BMC estimates: (1) small changes in hydration of FFM, and (2) body thickness do not affect estimates. Given the marked changes in body water in pregnancy, at parturition and postdelivery, DXA measurements are typically conducted a few weeks (range: 2–6 weeks17,48,75) after delivery to allow for some normalization of body water values. It is unclear, however, whether BMC changes between delivery and this period, particularly for lactating mothers, or whether varying subject thickness influences BMC values in postpartum women. Although DXA has been suggested for measuring total fat and FFM from prepregnancy to postpartum, as well as changes in regional lean and FM,64 we caution the use of DXA in the immediate postpartum period for estimating fat, FFM or lean mass due to the influences of ongoing changes in body water and hydration of FFM that likely violate the assumptions inherent in these estimates.
SUMMARY OF BODY COMPOSITION CHANGES IN PREGNANCY
Overall maternal body composition changes markedly across the course of pregnancy to support the growing fetus and prepare the mother for lactation. Here we summarize the results from several reports of four-compartment estimates of body composition changes in multi-ethnic women from metropolitan areas in the United States (Table 4). Overall, these women exhibited somewhat similar gains in weight, fat and body water across pregnancy, and subsequent decline in values postpartum. Whether women in other countries and socioeconomic settings or women with overweight/obesity/underweight prepregnancy BMI or excessive/ inadequate GWG experience similar changes in body composition over the course of pregnancy and postpartum is unclear and warrants further research.
Table 4.
Summary of four-compartment estimates of body composition across pregnancy
| Weeks |
||||||
|---|---|---|---|---|---|---|
| Prepregnancy | 8–14 | 26 | 36–40 | 2–6 Postpartum | ||
| Hopkinson et al.1,48 | ||||||
| n = 56 | Body weight, kg | 60.7 ±9.1 | 74.6 ± 10.1 | 66.3 ± 9.8 | ||
| Houston area | FM, kg | 22.8 ± 7.1 | 22.0 ± 7.3 | |||
| 0.6 mean parity | TBW, kg | 39.3 ± 3.9 | 33.2 ±3.8 | |||
| Multi-ethnic: predominately White | ||||||
| Kopp-Hoolihan et al.2,17 | ||||||
| n = 9 | Body weight, kg | 64.7 ± 7.8 | 64.9 ±8.4 | 72.1 ±9.4 | 75.9 ± 9.6 | 68.0 ± 8.9 |
| San Francisco Bay area | FM, kg | 46.3 ± 6.2 | 46.7 ±6.5 | 49.7 ± 5.8 | 52.8 ± 5.3 | 46.7 ±5.1 |
| Primi/multiparous | FFM, kg | 20.2 ± 4.7 | 20.3 ±4.7 | 24.4 ± 4.4 | 24.3 ± 5.6 | 22.0 ± 4.6 |
| Race/ethnicity not reported | TBW, kg | 33.5 ±4.5 | 33.9 ±4.8 | 36.5 ±4.1 | 39.1 ± 3.9 | 33.8 ±3.7 |
| Lederman et al.3,75 | ||||||
| n = 196 | Body weight, kg | 63.3 ± 12.9 | 65.3 ±12.9 | 76.8 ± 12.6 | ||
| New York area | FM, kg | 21.4 ±9.04 | 24.8 ± 8.58 | |||
| 0.8 mean parity | TBW, l | 32.4 ±4.7 | 39.4 ± 5.15 | |||
| Multi-ethnic: Black, White, Hispanic, and non-Hispanic | ||||||
Abbreviations: FM, fat mass; FFM, fat-free mass; TBW, total body weight. Values reported are mean ± s.d.
OVERVIEW OF PREDICTORS OF BODY COMPOSITION CHANGES IN PREGNANCY
Several factors, including initial size, parity, race and socioeconomic status, have been established as predictors of GWG;1 however, whether these factors independently predict overall body composition changes across pregnancy is unclear due to limited studies in this area. Here, we briefly review predictors of these changes, including initial maternal size, parity, socioeconomic factors and adolescent pregnancy. Initial maternal size, measured typically by prepregnancy BMI, is associated with regional36,76 and total body changes in fat but not TBW.15 Heavier women show smaller changes in SFT, whereas underweight women show larger changes.33,36 Primiparous women showed larger changes in skinfolds, compared with multiparous women.33,36 Compared with girls who had stopped growing or mature women, adolescent pregnancy among growing girls was associated with continued increases in arm fat area and no changes in subscapular SFT after 28-week gestation, suggesting that energy reserves were being conserved.77 There is some evidence of associations between higher socioeconomic status and regional changes in body composition;35 however, few studies have reported associations between socioeconomic status and overall changes in composition. Wealth, occupation, education, as well as, maternal age and parity predicted changes in midupper arm circumferences, arm muscle area or arm fat area in generally healthy HIV+ Malawian women, while seasonal exposure to famine modified some of these associations.35 Some but not all maternal and offspring obesity-related genetic variants are associated with GWG,78,79 but it is unknown whether these variants are associated with the composition of GWG. Although racial differences in regional fat distribution have been reported,80 these reports have not included pregnancy and it is unknown whether body composition changes during pregnancy differ by race/ethnicity.
OUTCOMES OF MATERNAL BODY COMPOSITION CHANGES IN PREGNANCY
Although there is a growing body of research focusing on perinatal, offspring and maternal outcomes of GWG,4 there is a dearth of information on the short- and long-term outcomes of body composition changes during pregnancy on offspring and maternal outcomes and also whether these associations vary by initial BMI and body composition (FM, FFM). This is largely attributable to challenges in measuring body composition during pregnancy. The composition of GWG has been identified as a predictor of postpartum weight and fat retention, as well as the pattern of adipose tissue accretion in parous versus nulliparous women. In 63 women from the Houston area, greater FM gains in pregnancy was associated with higher weight and fat retention at 27 weeks postpartum.12 Moreover, childbearing was associated with greater visceral adipose tissue accumulation, independent of overall body fat changes.81 Several studies have established that the overall composition of weight gain, specifically gains in body water and/or lean mass, are associated with greater offspring birth weight,12,75,82 whereas gains in fat are not associated with birth weight.12,18,75 Because of the limited studies with robust body composition estimates in pregnancy that have measurements beyond the first year of life, it is unclear whether the composition of GWG is associated with later maternal and offspring FM and obesity-associated sequelae.
RESEARCH GAPS IN BODY COMPOSITION ASSESSMENT IN PREGNANCY
Due to the dearth of literature in this area, we were unable to do a systematic review or meta-analysis on this subject. Few studies have been conducted in contemporary cohorts of multi-ethnic populations of varying initial size (for example, underweight, overweight or obese) and socioeconomic status. It is unclear whether racial and ethnic differences in body composition observed in women of reproductive age also apply to changes in body composition in pregnancy.80 Although several methods are considered ‘satisfactory’1 or ‘acceptable’27 to assess changes in body composition in pregnancy, all well-established methods of overall body composition are unable to disentangle the maternal- –fetal unit, which is an important goal for future research in this area; differentiating some components (for example, adipose tissue, skeletal muscle mass) may be feasible with MRI. It is clear that validation of methods that are non-invasive and readily accessible in clinical settings such as BIA and ultrasound appear warranted. If possible, validation of BIA is needed so that revised BIA equations and/or correction factors can be developed that account for changes in TBW and the FFM hydration in pregnancy. Ultrasound methodology needs to be standardized for use in clinical practice. Although several equations are available for estimating fat and lean mass in pregnancy, further development of equations that account for predictors of body composition that may vary across gestational age is needed. Finally, studies that examine determinants and outcomes of body composition changes in pregnancy are needed in order to guide future interventions and public health policies to optimize maternal health in pregnancy and maternal and offspring health postpartum.
CONCLUSIONS
Maternal body composition changes in pregnancy are associated with maternal and infant health outcomes in the immediate postpartum period and thereafter. Although there is a body of research regarding predictors and outcomes of GWG, these studies are unable to disentangle the components of weight gain (FM versus FFM) and how these components influence both maternal and offspring health. This may be due, in part, to the challenges of measuring maternal body composition in pregnancy due to the dynamic shifts in this period and also the lack of available methods to differentiate maternal and fetal components. The most commonly used method to measure maternal body composition changes in pregnancy is anthropometry (SFT and mid-upper arm circumferences). Although BIA has been used, it does not provide a measure of maternal body composition that is independent of the fetus and supporting tissues. The gold-standard four-compartment model currently provides the most robust estimates of body composition and changes in pregnancy but is unable to disentangle maternal and fetal tissues. Emerging methods that yet require validation and standardized methodology include MRI and ultrasound. Future research should focus on improving estimation of body composition changes and understanding predictors or modifiers of these changes, particularly prepregnancy BMI and body composition, across the duration of pregnancy in resource-rich and resource-poor settings with the ultimate goal of improving maternal and offspring health in pregnancy and thereafter.
ACKNOWLEDGEMENTS
This work was supported in part by P30-DK-26687, UO1-DK-094463, and UL1 TR000040. EW is supported by T32 DK091227.
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest.
REFERENCES
- 1.Institute of Medicine. Weight Gain During Pregnancy: Reexamining the Guidelines. Washington, DC, USA: National Academies Press; 2009. [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention. Pregnancy Nutrition Surveillance: Summary of Trends in Maternal Health Indicators by Race/Ethnicity 2012 [cited 12 November 2012] 2011 Available from: http://www.cdc.gov/PEDNSS/pnss_tables/pdf/national_table20.pdf.
- 3.Drehmer M, Duncan BB, Kac G, Schmidt MI. Association of second and third trimester weight gain in pregnancy with maternal and fetal outcomes. PLoS One. 2013;8:e54704. doi: 10.1371/journal.pone.0054704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Viswanathan M, Siega-Riz AM, Moos MK, Deierlein A, Mumford S, Knaack J, et al. Outcomes of maternal weight gain. Evid Rep Technol Assess (Full Rep) 2008;168:1–223. [PMC free article] [PubMed] [Google Scholar]
- 5.Margerison Zilko CE, Rehkopf D, Abrams B. Association of maternal gestational weight gain with short- and long-term maternal and child health outcomes. Am J Obstet Gynecol. 2010;202:574 e1–574 e8. doi: 10.1016/j.ajog.2009.12.007. [DOI] [PubMed] [Google Scholar]
- 6.Mamun AA, Kinarivala M, O'Callaghan MJ, Williams GM, Najman JM, Callaway LK. Associations of excess weight gain during pregnancy with long-term maternal overweight and obesity: evidence from 21 y postpartum follow-up. Am J Clin Nutr. 2010;91:1336–1341. doi: 10.3945/ajcn.2009.28950. [DOI] [PubMed] [Google Scholar]
- 7.Deierlein AL, Siega-Riz AM, Adair LS, Herring AH. Effects of pre-pregnancy body mass index and gestational weight gain on infant anthropometric outcomes. J Pediatr. 2011;158:221–226. doi: 10.1016/j.jpeds.2010.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hinkle SN, Sharma AJ, Swan DW, Schieve LA, Ramakrishnan U, Stein AD. Excess gestational weight gain is associated with child adiposity among mothers with normal and overweight prepregnancy weight status. J Nutr. 2012;142:1851–1858. doi: 10.3945/jn.112.161158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hull HR, Thornton JC, Ji Y, Paley C, Rosenn B, Mathews P, et al. Higher infant body fat with excessive gestational weight gain in overweight women. Am J Obstet Gynecol. 2011;205:211 e1–211 e7. doi: 10.1016/j.ajog.2011.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Popkin BM. The shift in stages of the nutrition transition in the developing world differs from past experiences! Public Health Nutr. 2002;5:205–214. doi: 10.1079/PHN2001295. [DOI] [PubMed] [Google Scholar]
- 11.Popkin BM. The nutrition transition: an overview of world patterns of change. Nutr Rev. 2004;62:S140–S143. doi: 10.1111/j.1753-4887.2004.tb00084.x. [DOI] [PubMed] [Google Scholar]
- 12.Butte NF, Ellis KJ, Wong WW, Hopkinson JM, Smith EO. Composition of gestational weight gain impacts maternal fat retention and infant birth weight. Am J Obstet Gynecol. 2003;189:1423–1432. doi: 10.1067/s0002-9378(03)00596-9. [DOI] [PubMed] [Google Scholar]
- 13.Bodnar LM, Siega-Riz AM, Simhan HN, Himes KP, Abrams B. Severe obesity, gestational weight gain, and adverse birth outcomes. Am J Clin Nutr. 2010;91:1642–1648. doi: 10.3945/ajcn.2009.29008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Forbes GB. Human Body Composition: Growth, Aging, Nutrition and Activity. New York, NY, USA: Springer-Verlag; [Google Scholar]
- 15.Lederman SA, Paxton A, Heymsfield SB, Wang J, Thornton J, Pierson RN., Jr Body fat and water changes during pregnancy in women with different body weight and weight gain. Obstet Gynecol. 1997;90:483–488. doi: 10.1016/s0029-7844(97)00355-4. [DOI] [PubMed] [Google Scholar]
- 16.Hytten FaC. GClinical Physiology in Obstetrics. Oxford, UK: Blackwell Scientfiic Publications; [Google Scholar]
- 17.Kopp-Hoolihan LE, van Loan MD, Wong WW, King JC. Fat mass deposition during pregnancy using a four-component model. J Appl Physiol. 1999;87:196–202. doi: 10.1152/jappl.1999.87.1.196. [DOI] [PubMed] [Google Scholar]
- 18.Forsum E, Sadurskis A, Wager J. Resting metabolic rate and body composition of healthy Swedish women during pregnancy. Am J Clin Nutr. 1988;47:942–947. doi: 10.1093/ajcn/47.6.942. [DOI] [PubMed] [Google Scholar]
- 19.van Raaij JM, Peek ME, Vermaat-Miedema SH, Schonk CM, Hautvast JG. New equations for estimating body fat mass in pregnancy from body density or total body water. Am J Clin Nutr. 1988;48:24–29. doi: 10.1093/ajcn/48.1.24. [DOI] [PubMed] [Google Scholar]
- 20.Catalano PM, Wong WW, Drago NM, Amini SB. Estimating body composition in late gestation: a new hydration constant for body density and total body water. Am J Physiol. 1995;268:E153–E158. doi: 10.1152/ajpendo.1995.268.1.E153. [DOI] [PubMed] [Google Scholar]
- 21.Fidanza F. The density of fat-free body mass during pregnancy. Int J Vitam Nutr Res. 1987;57:104. [PubMed] [Google Scholar]
- 22.Paxton A, Lederman SA, Heymsfield SB, Wang J, Thornton JC, Pierson RN., Jr Anthropometric equations for studying body fat in pregnant women. Am J Clin Nutr. 1998;67:104–110. doi: 10.1093/ajcn/67.1.104. [DOI] [PubMed] [Google Scholar]
- 23.Hutcheon JA, Platt RW, Abrams B, Himes KP, Simhan HN, Bodnar LM. A weight-gain-for-gestational-age z score chart for the assessment of maternal weight gain in pregnancy. Am J Clin Nutr. 2013;97:1062–1067. doi: 10.3945/ajcn.112.051706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lederman SA. Pregnancy. In: Heymsfield SB, Lohman TG, Wang Z, Going SB, editors. Human Body Composition. 2 edn. Champaign, IL, USA: Human Kinetics; 2005. pp. 299–312. [Google Scholar]
- 25.Hytten F. Weight gain in pregnancy. In: Hytten FE, Chamberlain G, editors. Clinical Physiology in Obstetrics Part 2: Nutrition and Metabolism. Oxford, UK: Blackwell Scientific Publications; 1980. pp. 193–233. [Google Scholar]
- 26.Institute of Medicine. Nutrition During Pregnancy: Part I Nutritional Status and Weight Gain. Washington, DC, USA: National Library of Medicine; 1990. [Google Scholar]
- 27.Food and Agriculture Organization/World Health Organization/United Nations University Human energy requirements: Report of a Joint FAO/WHO/UNU Expert Consultation. Rome, Italy: Available from: http://www.who.int/nutrition/publications/nutrientrequirements/9251052123/en/ [Google Scholar]
- 28.Sloan AW, Weir JB. Nomograms for prediction of body density and total body fat from skinfold measurements. J Appl Physiol. 1970;28:221–222. doi: 10.1152/jappl.1970.28.2.221. [DOI] [PubMed] [Google Scholar]
- 29.Steinkamp RC, Cohen NL, Gaffey WR, McKey T, Bron G, Siri WE, et al. Measures of body fat and related factors in normal adults. II. A simple clinical method to estimate body fat and lean body mass. J Chronic Dis. 1965;18:1291–1307. doi: 10.1016/0021-9681(65)90162-1. [DOI] [PubMed] [Google Scholar]
- 30.Durnin JV, Womersley J. Body fat assessed from total body density and its estimation from skinfold thickness: measurements on 481 men and women aged from 16 to 72 years. Br J Nutr. 1974;32:77–97. doi: 10.1079/bjn19740060. [DOI] [PubMed] [Google Scholar]
- 31.Jackson AS, Pollock ML, Ward A. Generalized equations for predicting body density of women. Med Sci Sports Exerc. 1980;12:175–181. [PubMed] [Google Scholar]
- 32.Huston Presley L, Wong WW, Roman NM, Amini SB, Catalano PM. Anthropometric estimation of maternal body composition in late gestation. Obstet Gynecol. 2000;96:33–37. doi: 10.1016/s0029-7844(00)00857-7. [DOI] [PubMed] [Google Scholar]
- 33.Taggart NR, Holliday RM, Billewicz WZ, Hytten FE, Thomson AM. Changes in skinfolds during pregnancy. Br J Nutr. 1967;21:439–451. doi: 10.1079/bjn19670045. [DOI] [PubMed] [Google Scholar]
- 34.Adair LS, Pollitt E, Mueller WH. Maternal anthropometric changes during pregnancy and lactation in a rural Taiwanese population. Hum Biol. 1983;55:771–787. [PubMed] [Google Scholar]
- 35.Ramlal RT, Tembo M, Soko A, Chigwenembe M, Tohill BC, Kayira D, et al. Patterns of body composition among HIV-infected, pregnant Malawians and the effects of famine season. Matern Child Health J. 2012;17:265–273. doi: 10.1007/s10995-012-0970-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sidebottom AC, Brown JE, Jacobs DR., Jr Pregnancy-related changes in body fat. Eur J Obstet Gynecol Reprod Biol. 2001;94:216–223. doi: 10.1016/s0301-2115(00)00329-8. [DOI] [PubMed] [Google Scholar]
- 37.Soltani H, Fraser RB. A longitudinal study of maternal anthropometric changes in normal weight, overweight and obese women during pregnancy and postpartum. Br J Nutr. 2000;84:95–101. doi: 10.1017/s0007114500001276. [DOI] [PubMed] [Google Scholar]
- 38.Forsum E, Sadurskis A, Wager J. Estimation of body fat in healthy Swedish women during pregnancy and lactation. Am J Clin Nutr. 1989;50:465–473. doi: 10.1093/ajcn/50.3.465. [DOI] [PubMed] [Google Scholar]
- 39.Robertson EG. Oedema in normal pregnancy. J Reprod Fertil Suppl. 1969;9:27–36. [PubMed] [Google Scholar]
- 40.Stevens-Simon C, Thureen P, Barrett J, Stamm E. Skinfold caliper and ultrasound assessments of change in the distribution of subcutaneous fat during adolescent pregnancy. Int J Obes Relat Metab Disord. 2001;25:1340–1345. doi: 10.1038/sj.ijo.0801685. [DOI] [PubMed] [Google Scholar]
- 41.Contreras Campos ME, Rodríguez-Cervantes N, Reza-López S, Ávila-Esparza M, Chávez-Corral DV, Levario-Carrillo M. Body composition and newborn birthweight in pregnancies of adolescent and mature women. Matern Child Nutr. 2012 Aug 22; doi: 10.1111/j.1740-8709.2012.00434.x. 2012; e-pub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sohlstrom A, Forsum E. Changes in total body fat during the human reproductive cycle as assessed by magnetic resonance imaging, body water dilution, and skinfold thickness: a comparison of methods. Am J Clin Nutr. 1997;66:1315–1322. doi: 10.1093/ajcn/66.6.1315. [DOI] [PubMed] [Google Scholar]
- 43.Lof M, Forsum E. Hydration of fat-free mass in healthy women with special reference to the effect of pregnancy. Am J Clin Nutr. 2004;80:960–965. doi: 10.1093/ajcn/80.4.960. [DOI] [PubMed] [Google Scholar]
- 44.Denne SC, Patel D, Kalhan SC. Total body water measurement in normal and diabetic pregnancy: evidence for maternal and amniotic fluid equilibrium. Biol Neonate. 1990;57:284–291. doi: 10.1159/000243203. [DOI] [PubMed] [Google Scholar]
- 45.Wang Z, Deurenberg P, Wang W, Pietrobelli A, Baumgartner RN, Heymsfield SB. Hydration of fat-free body mass: review and critique of a classic body-composition constant. Am J Clin Nutr. 1999;69:833–841. doi: 10.1093/ajcn/69.5.833. [DOI] [PubMed] [Google Scholar]
- 46.Prentice AM, Goldberg GR, Davies HL, Murgatroyd PR, Scott W. Energy-sparing adaptations in human pregnancy assessed by whole-body calorimetry. Br J Nutr. 1989;62:5–22. doi: 10.1079/bjn19890004. [DOI] [PubMed] [Google Scholar]
- 47.Lof M, Forsum E. Evaluation of bioimpedance spectroscopy for measurements of body water distribution in healthy women before, during, and after pregnancy. J Appl Physiol. 2004;96:967–973. doi: 10.1152/japplphysiol.00900.2003. [DOI] [PubMed] [Google Scholar]
- 48.Hopkinson JM, Butte NF, Ellis KJ, Wong WW, Puyau MR, Smith EO. Body fat estimation in late pregnancy and early postpartum: comparison of two-, three-, and four-component models. Am J Clin Nutr. 1997;65:432–438. doi: 10.1093/ajcn/65.2.432. [DOI] [PubMed] [Google Scholar]
- 49.Dempster P, Aitkens S. A new air displacement method for the determination of human body composition. Med Sci Sports Exerc. 1995;27:1692–1697. [PubMed] [Google Scholar]
- 50.McCrory MA, Gomez TD, Bernauer EM, Mole PA. Evaluation of a new air displacement plethysmograph for measuring human body composition. Med Sci Sports Exerc. 1995;27:1686–1691. [PubMed] [Google Scholar]
- 51.American College of Radiology Practice guidelines for the safe and optimal performance of fetal magnetic resonance imaging. [cited 2013 May 10, 2013]. Available from: http://www.acr.org/~/media/ACR/Documents/PGTS/guidelines/ MRI_Fetal.pdf.
- 52.Sohlstrom A, Forsum E. Changes in adipose tissue volume and distribution during reproduction in Swedish women as assessed by magnetic resonance imaging. Am J Clin Nutr. 1995;61:287–295. doi: 10.1093/ajcn/61.2.287. [DOI] [PubMed] [Google Scholar]
- 53.Sohlstrom A, Wahlund LO, Forsum E. Total body fat and its distribution during human reproduction as assessed by magnetic resonance imaging. Basic Life Sci. 1993;60:181–184. doi: 10.1007/978-1-4899-1268-8_41. [DOI] [PubMed] [Google Scholar]
- 54.Wells JC, Douros I, Fuller NJ, Elia M, Dekker L. Assessment of body volume using three-dimensional photonic scanning. Ann NY Acad Sci. 2000;904:247–254. doi: 10.1111/j.1749-6632.2000.tb06460.x. [DOI] [PubMed] [Google Scholar]
- 55.Wang J, Gallagher D, Thornton JC, Yu W, Horlick M, Pi-Sunyer FX. Validation of a 3-dimensional photonic scanner for the measurement of body volumes, dimensions, and percentage body fat. Am J Clin Nutr. 2006;83:809–816. doi: 10.1093/ajcn/83.4.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wells JC, Griffin L, Treleaven P. Independent changes in female body shape with parity and age: A life-history approach to female adiposity. Am J Hum Biol. 2010 Aug;22:456–462. doi: 10.1002/ajhb.21017. [DOI] [PubMed] [Google Scholar]
- 57.Wells JC, Charoensiriwath S, Treleaven P. Reproduction, aging, and body shape by three-dimensional photonic scanning in Thai men and women. Am J Hum Biol. 2011;23:291–298. doi: 10.1002/ajhb.21151. [DOI] [PubMed] [Google Scholar]
- 58.Wells JC. The thrifty phenotype: an adaptation in growth or metabolism? Am J Hum Biol. 2011;23:65–75. doi: 10.1002/ajhb.21100. [DOI] [PubMed] [Google Scholar]
- 59.Bartha JL, Marin-Segura P, Gonzalez-Gonzalez NL, Wagner F, Aguilar-Diosdado M, Hervias-Vivancos B. Ultrasound evaluation of visceral fat and metabolic risk factors during early pregnancy. Obesity (Silver Spring) 2007;15:2233–2239. doi: 10.1038/oby.2007.265. [DOI] [PubMed] [Google Scholar]
- 60.Kinoshita T, Itoh M. Longitudinal variance of fat mass deposition during pregnancy evaluated by ultrasonography: the ratio of visceral fat to subcutaneous fat in the abdomen. Gynecol Obstet Invest. 2006;61:115–118. doi: 10.1159/000089456. [DOI] [PubMed] [Google Scholar]
- 61.Fanelli MT, Kuczmarski RJ. Ultrasound as an approach to assessing body composition. Am J Clin Nutr. 1984;39:703–709. doi: 10.1093/ajcn/39.5.703. [DOI] [PubMed] [Google Scholar]
- 62.Armellini F, Zamboni M, Rigo L, Todesco T, Bergamo-Andreis IA, Procacci C, et al. The contribution of sonography to the measurement of intra-abdominal fat. J Clin Ultrasound. 1990;18:563–567. doi: 10.1002/jcu.1870180707. [DOI] [PubMed] [Google Scholar]
- 63.Deurenberg P, van der Kooy K, Leenen R, Schouten FJ. Body impedance is largely dependent on the intra- and extra-cellular water distribution. Eur J Clin Nutr. 1989;43:845–853. [PubMed] [Google Scholar]
- 64.McCarthy EA, Strauss BJ, Walker SP, Permezel M. Determination of maternal body composition in pregnancy and its relevance to perinatal outcomes. Obstet Gynecol Surv. 2004;59:731–742. doi: 10.1097/01.ogx.0000140039.10861.91. quiz 45–6. [DOI] [PubMed] [Google Scholar]
- 65.Sowers M, Crutchfield M, Jannausch M, Updike S, Corton G. A prospective evaluation of bone mineral change in pregnancy. Obstet Gynecol. 1991;77:841–845. [PubMed] [Google Scholar]
- 66.Ritchie LD, Fung EB, Halloran BP, Turnlund JR, Van Loan MD, Cann CE, et al. A longitudinal study of calcium homeostasis during human pregnancy and lactation and after resumption of menses. Am J Clin Nutr. 1998;67:693–701. doi: 10.1093/ajcn/67.4.693. [DOI] [PubMed] [Google Scholar]
- 67.Prentice A. Maternal calcium requirements during pregnancy and lactation. Am J Clin Nutr. 1994;59:477S–482S. doi: 10.1093/ajcn/59.2.477S. discussion 82S–83S. [DOI] [PubMed] [Google Scholar]
- 68.Christiansen C, Rodbro P, Heinild B. Unchanged total body calcium in normal human pregnancy. Acta Obstet Gynecol Scand. 1976;55:141–143. doi: 10.3109/00016347609156802. [DOI] [PubMed] [Google Scholar]
- 69.Naylor KE, Iqbal P, Fledelius C, Fraser RB, Eastell R. The effect of pregnancy on bone density and bone turnover. J Bone Miner Res. 2000;15:129–137. doi: 10.1359/jbmr.2000.15.1.129. [DOI] [PubMed] [Google Scholar]
- 70.Black AJ, Topping J, Durham B, Farquharson RG, Fraser WD. A detailed assessment of alterations in bone turnover, calcium homeostasis, and bone density in normal pregnancy. J Bone Miner Res. 2000;15:557–563. doi: 10.1359/jbmr.2000.15.3.557. [DOI] [PubMed] [Google Scholar]
- 71.Lamke B, Brundin J, Moberg P. Changes of bone mineral content during pregnancy and lactation. Acta Obstet Gynecol Scand. 1977;56:217–219. doi: 10.3109/00016347709162123. [DOI] [PubMed] [Google Scholar]
- 72.Drinkwater BL, Chesnut CH., 3rd Bone density changes during pregnancy and lactation in active women: a longitudinal study. Bone Miner. 1991;14:153–160. doi: 10.1016/0169-6009(91)90092-e. [DOI] [PubMed] [Google Scholar]
- 73.Olausson H, Laskey MA, Goldberg GR, Prentice A. Changes in bone mineral status and bone size during pregnancy and the influences of body weight and calcium intake. Am J Clin Nutr. 2008;88:1032–1039. doi: 10.1093/ajcn/88.4.1032. [DOI] [PubMed] [Google Scholar]
- 74.Moller UK, Vieth Streym S, Mosekilde L, Rejnmark L. Changes in bone mineral density and body composition during pregnancy and postpartum. A controlled cohort study. Osteoporos Int. 2012;23:1213–1223. doi: 10.1007/s00198-011-1654-6. [DOI] [PubMed] [Google Scholar]
- 75.Lederman SA, Paxton A, Heymsfield SB, Wang J, Thornton J, Pierson RN., Jr Maternal body fat and water during pregnancy: do they raise infant birth weight? Am J Obstet Gynecol. 1999. 180:235–240. doi: 10.1016/s0002-9378(99)70181-x. [DOI] [PubMed] [Google Scholar]
- 76.Ehrenberg HM, Huston-Presley L, Catalano PM. The influence of obesity and gestational diabetes mellitus on accretion and the distribution of adipose tissue in pregnancy. Am J Obstet Gynecol. 2003;189:944–948. doi: 10.1067/s0002-9378(03)00761-0. [DOI] [PubMed] [Google Scholar]
- 77.Scholl TO, Hediger ML, Schall JI, Khoo CS, Fischer RL. Maternal growth during pregnancy and the competition for nutrients. Am J Clin Nutr. 1994;60:183–188. doi: 10.1093/ajcn/60.2.183. [DOI] [PubMed] [Google Scholar]
- 78.Lawlor DA, Fraser A, Macdonald-Wallis C, Nelson SM, Palmer TM, Davey Smith G, et al. Maternal and offspring adiposity-related genetic variants and gestational weight gain. Am J Clin Nutr. 2011;94:149–155. doi: 10.3945/ajcn.110.010751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Stuebe AM, Lyon H, Herring AH, Ghosh J, Wise A, North KE, et al. Obesity and diabetes genetic variants associated with gestational weight gain. Am J Obstet Gynecol. 2010;203:283, e1–e17. doi: 10.1016/j.ajog.2010.06.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rahman M, Temple JR, Breitkopf CR, Berenson AB. Racial differences in body fat distribution among reproductive-aged women. Metabolism. 2009;58:1329–1337. doi: 10.1016/j.metabol.2009.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gunderson EP, Sternfeld B, Wellons MF, Whitmer RA, Chiang V, Quesenberry CP, Jr, et al. Childbearing may increase visceral adipose tissue independent of overall increase in body fat. Obesity (Silver Spring) 2008;16:1078–1084. doi: 10.1038/oby.2008.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gernand AD, Christian P, Paul RR, Shaikh S, Labrique AB, Schulze KJ, et al. Maternal weight and body composition during pregnancy are associated with placental and birth weight in rural Bangladesh. J Nutr. 2012;142:2010–2016. doi: 10.3945/jn.112.163634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Siri WE. Body composition from fluid spaces and density: an analysis of method. In: Brozek J, Henschel A, editors. Techniques for Measuring Body Composition. Washington, DC, USA: National Academy of Science National Research Council; 1961. pp. 223–244. [Google Scholar]
- 84.Garrow JS. Indicies of adiposity. Nutr Abst Rev Clin Nutr Series. 1983;53:697–708. [Google Scholar]
- 85.Brozek J, Grande F, Anderson JT, Keys A. Densitometric analysis of body composition: revision of some quantitative assumptions. Ann NY Acad Sci. 1963;110:113–140. doi: 10.1111/j.1749-6632.1963.tb17079.x. [DOI] [PubMed] [Google Scholar]
- 86.Selinger A. The body as a three component system (PhD) Ann Arbor, MI, USA: University of Illinois at Urbana-Champaign; 1977. [Google Scholar]
- 87.Goldman D, Buskirk ER. A method for underwater weighing and the determination of body density. In: Brozel J, Henschel A, editors. Techniques for Measuring Body Composition. Washington, DC, USA: National Academy of Sciences; 1961. pp. 78–106. [Google Scholar]