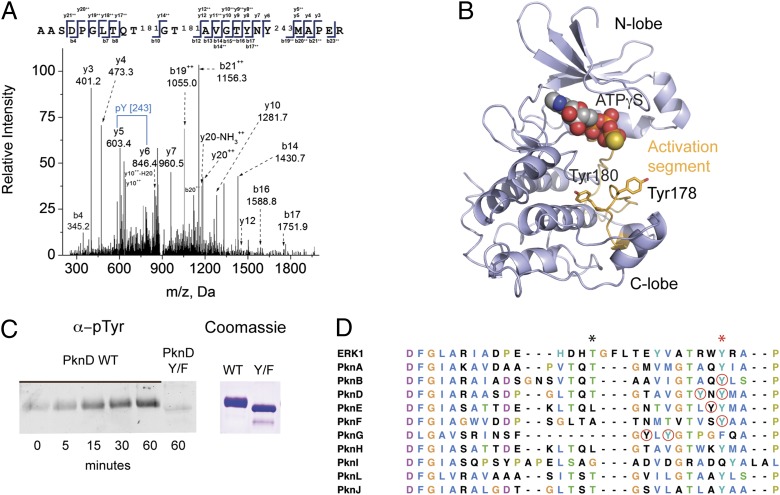
Fig. 1.
STPKs are dual-specificity kinases. (A) MS/MS spectrum showing phosphorylation of recombinant PknD KD on Tyr180. (B) A prediction of the PknD structure (PHYRE) shows the predicted position of pTyr178 and -180 on the activation segment (orange) relative to the ATP-binding site, N lobe, and C lobe. (C) Autophosphorylation of recombinant PknD KD on Tyr. Western blotting with anti-pTyr antibody shows an increase in PknD Tyr phosphorylation after incubation with ATP and Mn2+ and loss of Tyr phosphorylation in the Tyr(178,180)Phe double mutant, showing intrinsic Tyr kinase activity. (D) Sequence alignment of Mtb STPK activation segments. Red circles indicate pTyr sites identified in this study. The black asterisk marks the position of phospho-amino acids that regulate activity in some Mtb STPKs, and a red asterisk marks conserved Tyr.