Abstract
Repair of bone erosions in rheumatoid arthritis has been considered a difficult goal to achieve. However—with better therapies at hand to control synovial inflammation—sensitive μCT imaging techniques now available confirm that repair of bone erosion is possible, and begins at the base of erosive lesions.
The past decade has seen remarkable progress in the treatment of rheumatoid arthritis (RA), accompanied by an increasing interest in the mechanisms responsible for destruction of the articular bone in this disease. Inflammation in the bone micro-environment in arthritic conditions such as RA leads to the generation of bone-resorbing osteoclasts, and, at the same time, bone formation by osteoblasts is suppressed, resulting in a net loss of bone and impaired capacity for erosion repair. Nevertheless, despite the use of potent anti-inflammatory agents such as TNF inhibitors—which control clinical inflamma tion and specifically block cytokines that mediate inflamma tion—repair of eroded articular bone seems, perplexingly, to occur infrequently. The explanation for this intriguing clinical observation remains to be fully elucidat ed. A report by Finzel et al.1 in Annals of the Rheumatic Diseases now offers insights into the capacity of tradi tional and biologic therapies to promote repair of erosions in patients with RA. Their work suggests that the use of TNF inhibitors moves us in the right direction in fostering bone formation, but complete repair of erosions remains an elusive clinical goal.
Finzel and colleagues used high-resolution micro-computed tomography (μCT) scanning to better evaluate the impact of RA therapy on repair of articular bone. Erosions detec ted in patients undergoing metho trexate therapy alone, or metho trexate plus an anti-TNF agent (after a minimum of 3 months of stable treatment), were measured for width and depth at base line and after 1 year of treatment. Using this sensitive μCT technique, the authors showed a statistically significant improve ment in the mean depth, but not width, of erosions in patients receiving anti-TNF therapy. By contrast, erosions in patients treated with methotrexate alone showed an increase in mean erosion width and depth, indicating progression. Ero sions that showed a reduction in depth were typically deep lesions, characterized by the presence of sclero tic bone either at baseline and/or at follow-up, indicating that bone repair resulted from new bone formation on endosteal surfaces at the base of the erosions.1
These data in patients with RA follow nicely from studies using mouse models of the disease, which examined the impact of inflammation on osteoblast maturation and bone formation in articular erosions. In these studies, bone surfaces adjacent to invading inflammatory tissue were found to be characterized by impaired bone formation associated with a paucity of mature osteo-blasts, despite the prevalence of immature osteoblast-lineage cells lining the endo steal bone surfaces.2 By contrast, as inflam mation resolved, mature osteo blasts popu lated these surfaces and formed bone, leading to repair of erosions over time.3 Therefore, the degree of local inflammation is likely to be an important factor in de termining erosion repair in RA.
Consistent with these observations, previous studies in patients with RA designed to identify repair of erosions by conventional radiography have demonstrated that erosion repair can occur;4 however, rates of repair are low. In a small cohort of patients with RA treated with conventional DMARD therapy, Ideguchi et al.5 demonstrated that repair occurred in approximately 10% of patients. Furthermore, repair was identified primarily in those patients with low disease activity, a finding supported in a more recent study of patients treated with conventional therapy.6
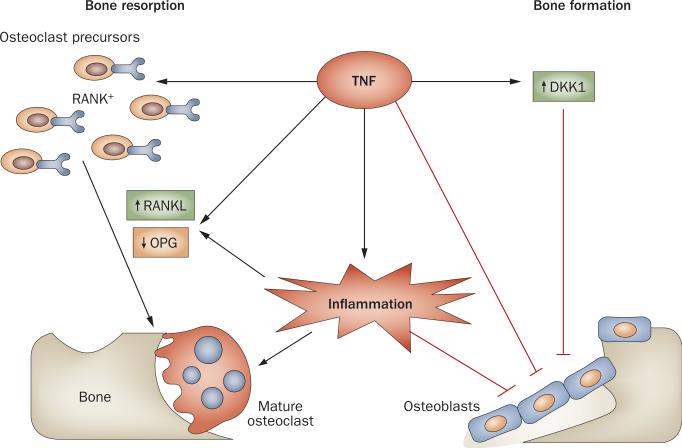
Why might we expect patients receiving anti-TNF therapy to experience a better rate of erosion repair than those treated with conventional DMARDs? TNF is a pro inflammatory cytokine that contributes significantly to the inflammatory process in RA. Moreover, TNF has an impact on several mechanisms that are directly involved in the erosive process (Figure 1): it increases the expression of the key osteoclast differentiation factor RANKL (receptor activator of nuclear factor κB-ligand, also known as TNF ligand super family member 11); it expands the pool of osteoclast pre cursor cells; and it can act synergisti cally with RANKL to promote osteoclast differ entiation.7 In addition to promoting osteoclastogenesis and bone resorption, TNF also acts to inhibit bone formation by suppressing osteoblast matura tion through inhibition of the expression of runt-related transcription factor 2, a trans cription factor critical for osteoblastlineage commitment and gene expression, resulting in reduced bone forma tion and mineraliza tion.7 Furthermore, TNF can act on synovial fibroblasts from patients with RA to induce the expression of Dickkopf-1, an inhibitor of the Wnt signaling pathway.8 This pathway is essential for osteoblast differentiation, and for the bone-forming function of these cells. It therefore follows that aggressive treatment of inflammation, and in particular, blockade of TNF, could support the repair of articular erosions.
Figure 1.
TNF—a multipotent cytokine in RA. TNF acts in proinflammatory signaling pathways within the joint to contribute to inflammation, and has synergistic effects on bone turnover: it promotes osteoclast-mediated bone erosion by expanding the RANK+ osteoclast precursor cell population and promoting expression of RANKL relative to OPG; it inhibits osteoblast maturation and function, in part by increasing expression of the Wnt antagonist DKK1, resulting in impaired bone formation. Abbreviations: DKK1, Dickkopf-1; OPG, osteoprotegerin (also known as TNF receptor superfamily member 11B); RANK, receptor activator of nuclear factor κB; RANKL, RANK ligand.
The evidence provided by Finzel et al.1 indeed demonstrates that the addition of an anti-TNF agent to methotrexate therapy improves the likelihood of erosion repair, compared with methotrexate treatment alone. These findings are supportive of other studies published recently, in which a variety of radiographic techniques were used to assess bone, and which demonstrated improvement in erosions following treatment with an anti-TNF agent.9,10 Interestingly, in the current study1 a greater percentage of erosions showed signs of sclerosis—indicating some degree of ongoing bone formation —at baseline in patients treated with methotrexate alone, compared with patients treated with the addition of an anti-TNF agent. This observation might reflect that these patients had less severe disease than those in whom the addition of TNF blockade was required.
Although the study by Finzel et al.1 is encouraging in that it shows that TNF inhibitors might be able to reverse erosive damage to a greater degree than has previously been surmised, repair is limited. The μCT scanning used by the investigators is excellent for quantitation of bone, but it does not allow for assessment of synovial inflammation or of bone marrow edema, as was accomplished in a previous study.10 Therefore, the question of whether inflamma tion was eradicated at sites of bone erosion that did or did not repair could not be addressed. Additional studies using μCT in conjunction with MRI or sensitive ultrasound techniques that can accurately measure residual synovial inflammation within individual sites of erosion could address the remaining important question: is repair of erosions directly related to the ability of therapeutic interventions to strictly control local joint inflammation?
Finally, how important is it in the clinical care of patients with RA that articular bone erosions heal? Strong evidence certainly exists for the correlation of bone erosion and joint damage with functional disability over time in these patients, but we do not yet know the functional implications of erosion repair. Nonetheless, if a direct correlation between local control of inflammation and repair of erosions were to be clearly established in patients, then repair might become an important indicator of therapeutic efficacy. The use of animal models of RA will be important to further elucidate the molecular pathways that are active at sites of erosion, and that create a local micro environment conducive for bone repair.
In summary, Finzel et al.1 have clearly shown that repair of bone erosions can occur in patients with RA, and is character ized by formation of new bone at the base of deep erosions. Control of inflammation, which is likely to be key in shifting the balance towards bone formation and facilitating healing of erosions, remains an important clinical goal.
“...addition of an anti-TNF agent to methotrexate therapy improves the likelihood of erosion repair”
Acknowledgments
The work of E. M. Gravallese is supported by NIH grant AR055952. The work of N. C. Walsh is supported by an Australian National Health and Medical Research Council project grant.
Footnotes
Competing interests The authors declare no competing interests.
References
- 1.Finzel S, et al. Repair of bone erosions in rheumatoid arthritis treated with tumour necrosis factor inhibitors is based on bone apposition at the base of the erosion. Ann. Rheum. Dis. 2011;70:1587–1593. doi: 10.1136/ard.2010.148395. [DOI] [PubMed] [Google Scholar]
- 2.Walsh NC, et al. Osteoblast function is compromised at sites of focal bone erosion in inflammatory arthritis. J. Bone Miner. Res. 2009;24:1572–1585. doi: 10.1359/jbmr.090320. [DOI] [PubMed] [Google Scholar]
- 3.Matzelle MM, et al. Repair of bone erosions in inflammatory arthritis occurs as inflammation resolves, accompanied by alterations in the Wnt signaling pathway [abstract 716]. Arthritis Rheum. 2010;62(Suppl.):S298. [Google Scholar]
- 4.Sharp JT, et al. Repair of erosions in rheumatoid arthritis does occur. Results from 2 studies by the OMERACT subcommittee on healing of erosions. J. Rheumatol. 2003;30:1102–1107. [PubMed] [Google Scholar]
- 5.Ideguchi H, Ohno S, Hattori H, Senuma A, Ishigatsubo Y. Bone erosions in rheumatoid arthritis can be repaired through reduction in disease activity with conventional disease-modifying antirheumatic drugs. Arthritis Res. Ther. 2006;8:R76. doi: 10.1186/ar1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van der Linden MP, et al. Repair of joint erosions in rheumatoid arthritis: prevalence and patient characteristics in a large inception cohort. Ann. Rheum. Dis. 2010;69:727–729. doi: 10.1136/ard.2009.108332. [DOI] [PubMed] [Google Scholar]
- 7.Walsh NC, Gravallese EM. Bone remodeling in rheumatic diseases: a question of balance. Immunol. Rev. 2010;233:301–312. doi: 10.1111/j.0105-2896.2009.00857.x. [DOI] [PubMed] [Google Scholar]
- 8.Diarra D, et al. Dickkopf-1 is a master regulator of joint remodeling. Nat. Med. 2007;13:156–163. doi: 10.1038/nm1538. [DOI] [PubMed] [Google Scholar]
- 9.Lukas C, van der Heijde D, Fatenajad S, Landewé R. Repair of erosions occurs almost exclusively in damaged joints without swelling. Ann. Rheum. Dis. 2010;69:851–855. doi: 10.1136/ard.2009.119156. [DOI] [PubMed] [Google Scholar]
- 10.Døhn UM, et al. No overall progression and occasional repair of erosions despite persistent inflammation in adalimumab-treated rheumatoid arthritis patients: results from a longitudinal comparative MRI, ultrasonography, CT and radiography study. Ann. Rheum. Dis. 2011;70:252–258. doi: 10.1136/ard.2009.123729. [DOI] [PubMed] [Google Scholar]