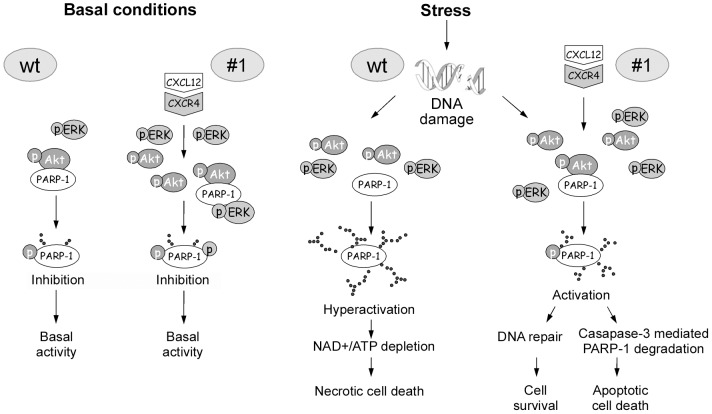
Figure 7. Antinecrotic effects of CXCL12 in pancreatic β-cells.
During the basal conditions in wt Rin-5F cells, PARP-1 interacts with activated pAkt. This interaction allows for PARP-1 phosphorylation that results in PARP-1 partial inhibition. In the increased presence of CXCL12 (#1) activated kinases, pAkt and pERK1/2 are found to be PARP-1 interacting partners with no additional influence on its enzymatic activity. In contrast, during the stress conditions and in response to severe DNA damage in wt cells, the loss of pAkt-PARP-1 interaction lifts the suppression on PARP-1 enzymatic activity, allowing for extensive PARP-1 auto-poly(ADP-ribosyl)ation, NAD+ and ATP depletion and final necrotic cell death. The excess of CXCL12 in #1 cells allows for the increased phosphorylation of Akt kinase and pAkt-PARP-1 interaction. This interaction persists even after an insult has passed, maintaining a continual partial suppression of overactivated PARP-1 that results in moderately active PARP-1. In this scenario, cellular energy depletion has been prevented and switch from the necrotic to the apoptotic death has been secured by pAkt-induced prevention of PARP-1 overactivation. With the fine-tuned suppression of overactivated PARP-1 in cell under the stress, cell still operates with the active PARP-1 that is essential player in many cellular processes.